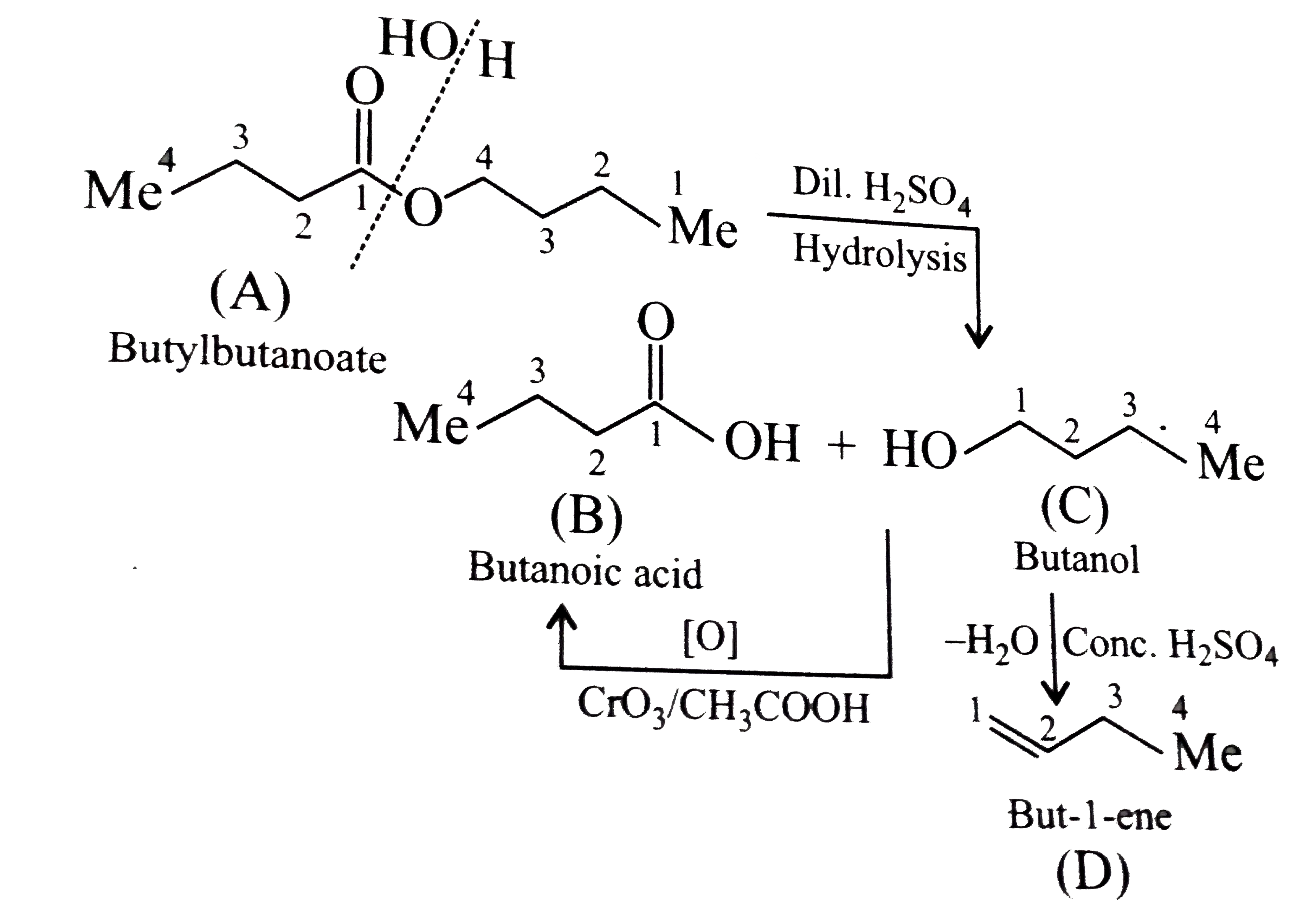
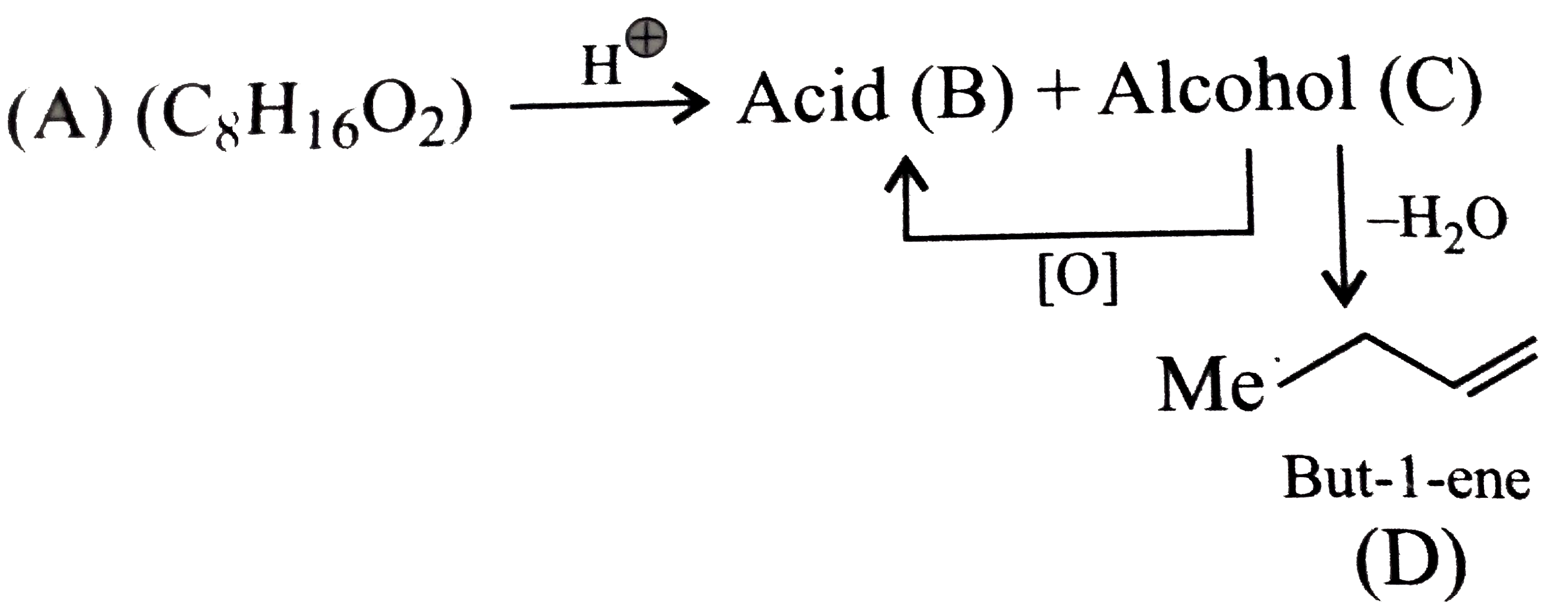Text Solution
Verified by Experts
Topper's Solved these Questions
ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|59 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|52 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|15 VideosAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type|179 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ALIPHATIC AND AROMATIC ALDEHYDES AND KETONES-Exercises Concept Application
- What is meant by the following terms ? Give an example of the reaction...
Text Solution
|
- Name the following compounds according to IUPAC system of nomenclature...
Text Solution
|
- Draw the structures of following compound: i. 3-Methylbutanal ii. ...
Text Solution
|
- Write the IUPAC names of following ketones and aldehydes. Wherever pos...
Text Solution
|
- Draw the structure of following derivatives: i. 2,4-Dinitrophylhydra...
Text Solution
|
- Predict the products formed when cyclohexanecarbaldehyde reacts with f...
Text Solution
|
- Which of the following compounds would undergo aldol condensation or t...
Text Solution
|
- How will you convert ethnal into the following compounds ? i. Butane...
Text Solution
|
- Write structure formulae and names of four possible aldol condensation...
Text Solution
|
- An organic compound (X) with molecular formula C(9)H(10)O gives positi...
Text Solution
|
- An organic compound (A) (molecular formula C(8)H(16)O(2)) was hydrolys...
Text Solution
|
- Arrange the following compounds in the increasing order of their prope...
Text Solution
|
- Give chemical tests to distinguish between the following pairs of comp...
Text Solution
|
- How will you prepare the following compounds from benzene? You may use...
Text Solution
|
- How will you bring about the following conversions in not more than tw...
Text Solution
|
- Describe the following i. Acetylation ii. Cannizzaro reaction i...
Text Solution
|
- Complete each synthesis by giving the missing starting material, reage...
Text Solution
|
- Give explanation of following : (i) During the preparation of esters...
Text Solution
|
- (a) Give chemical tests to distinguish between compounds in the follo...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|