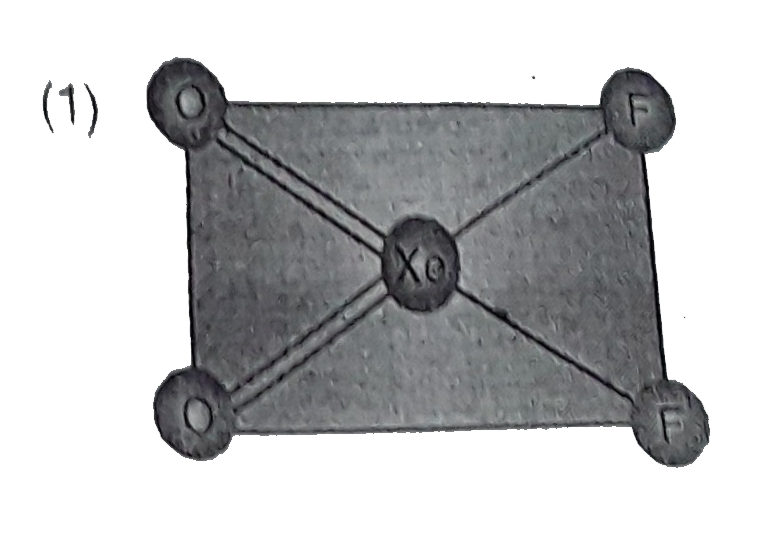
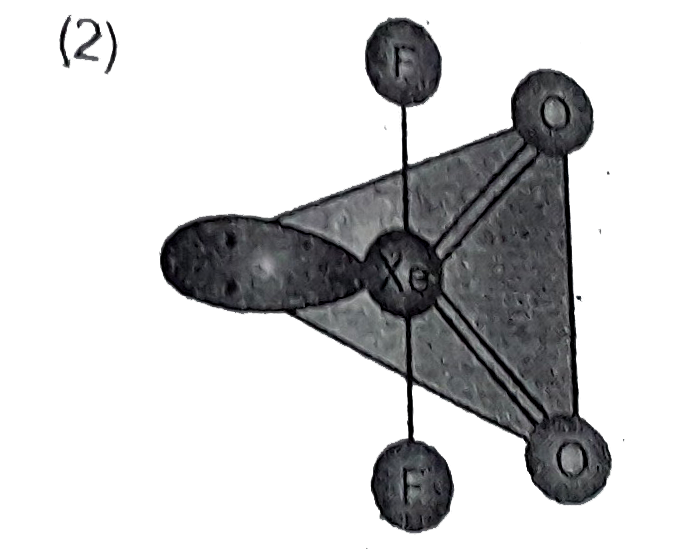
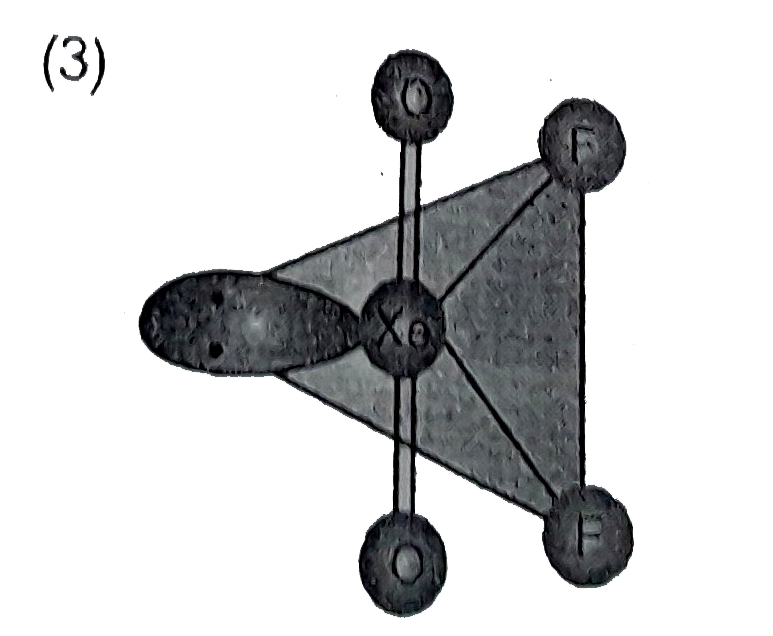
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section-B)|26 VideosTHE P-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment Section-C)|23 VideosTHE P-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Try yourself|70 VideosTHE D AND F-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assingment ( Section-J Aakash Challengers Questions )|10 VideosTHE S-BLOCK ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section-J)|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THE P-BLOCK ELEMENTS-Assignment Section-A)
- Which is incorrectly given according to order indicated?
Text Solution
|
- Cl(2) on reaction with excess of NH(3) gives
Text Solution
|
- Complete the following chemical reaction equations : (i) underset(("...
Text Solution
|
- Cl(2)+underset((Excess))(F(2)) overset(573K)to(A) Shape of compound ...
Text Solution
|
- The correct order of acidic strength is
Text Solution
|
- The one with maximum oxidising power is
Text Solution
|
- Total number of F-l-F bond angles which are 90^(@) present in lF(7) is
Text Solution
|
- Assertion : Interhalogen compounds are more reactive than halogens (ex...
Text Solution
|
- Cl(2) is used in the preparation of poisonous gases, one of them is mu...
Text Solution
|
- Anamolous behaviour of fluorine in group 17 is due to
Text Solution
|
- .(88)^(226)Ra to .(Z)^(A)Rn+.(2)^(4)He Radon is prepared by alpha-de...
Text Solution
|
- Noble gases are mostly inert. Assign reasons.
Text Solution
|
- underset((1:20))(Xe+F(2)) overset(573K,60-70" bar")to? The compound ...
Text Solution
|
- Why is helium used in diving apparatus?
Text Solution
|
- Which is mismatched regarding the shape?
Text Solution
|
- Compound of which of the noble gases, are neither isolated nor identif...
Text Solution
|
- Structure of XeO(2)F(2) is correctly represented by
Text Solution
|
- Why has it been difficult to study the chemistry of radon?
Text Solution
|
- The ease of liquefaction of noble gases decreases in the order
Text Solution
|
- Noble gases are sparingly soluble in water due to
Text Solution
|