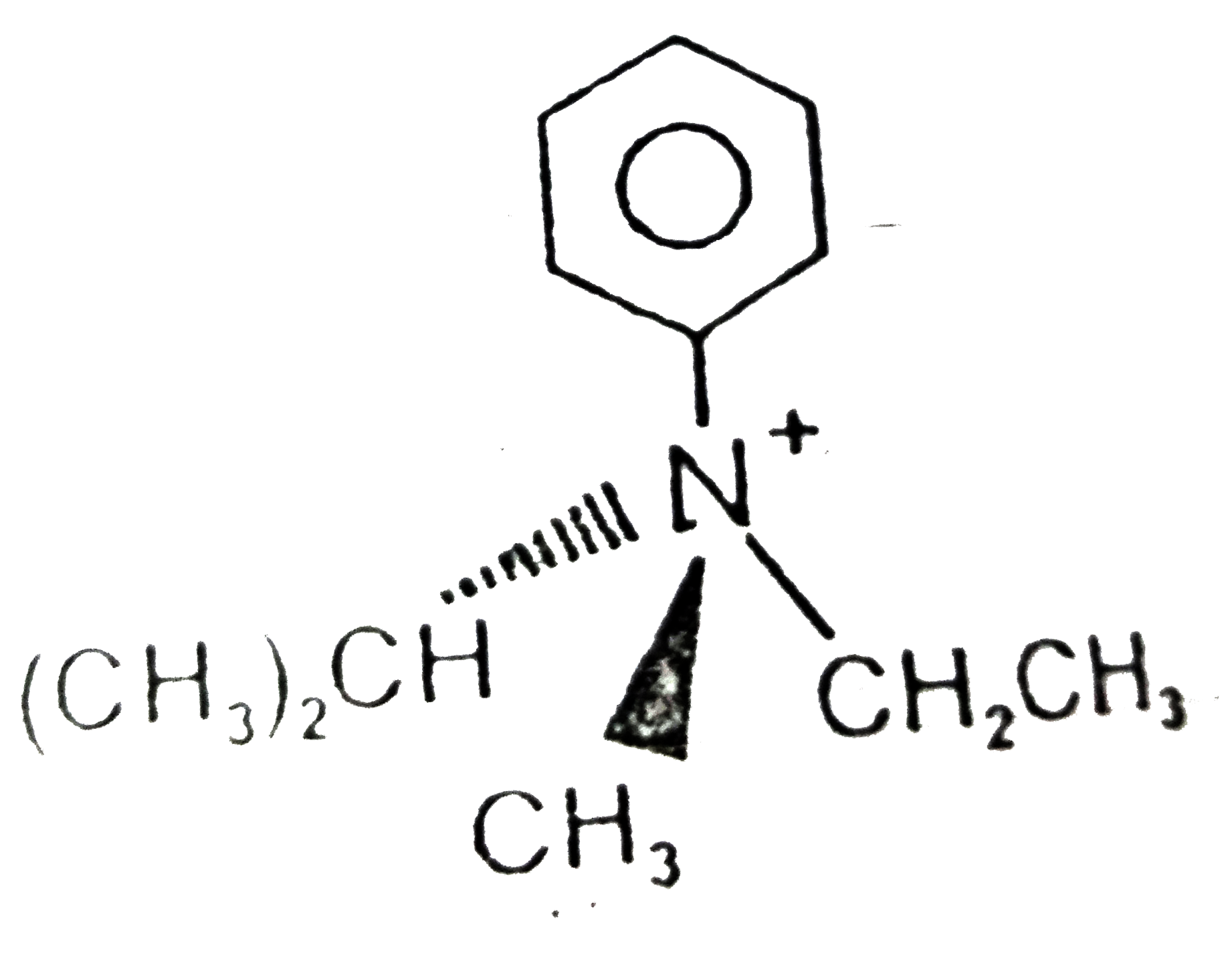
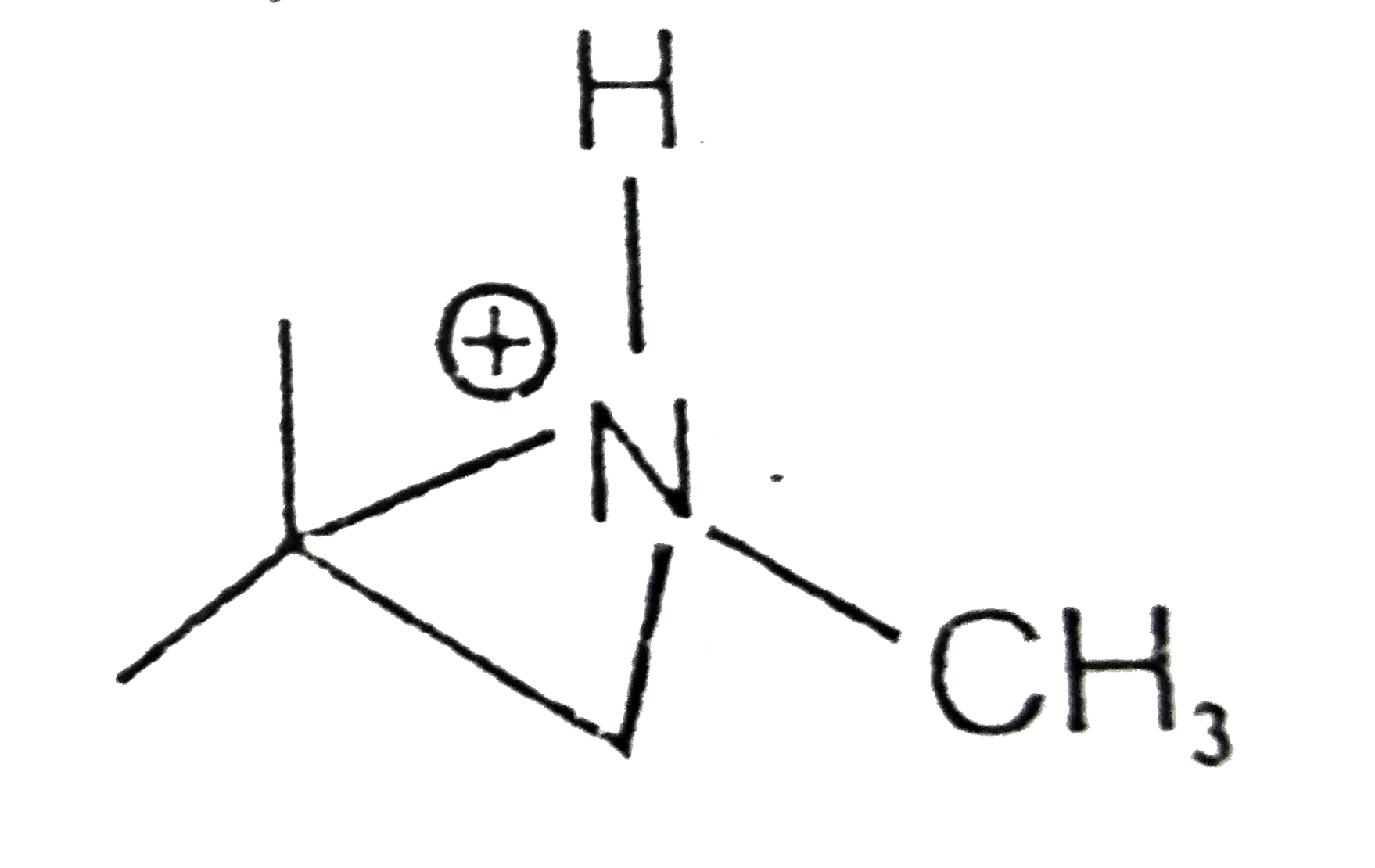
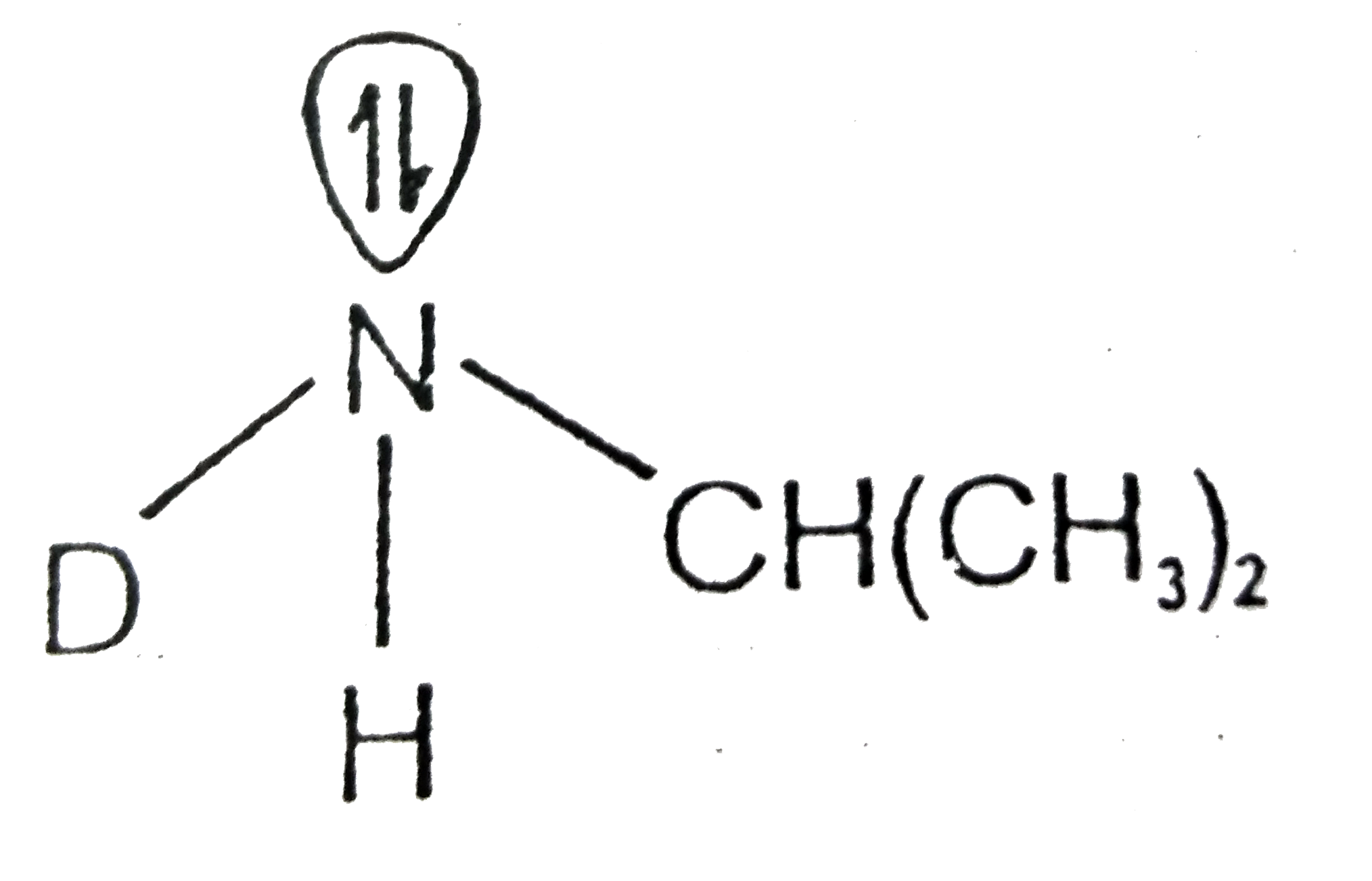
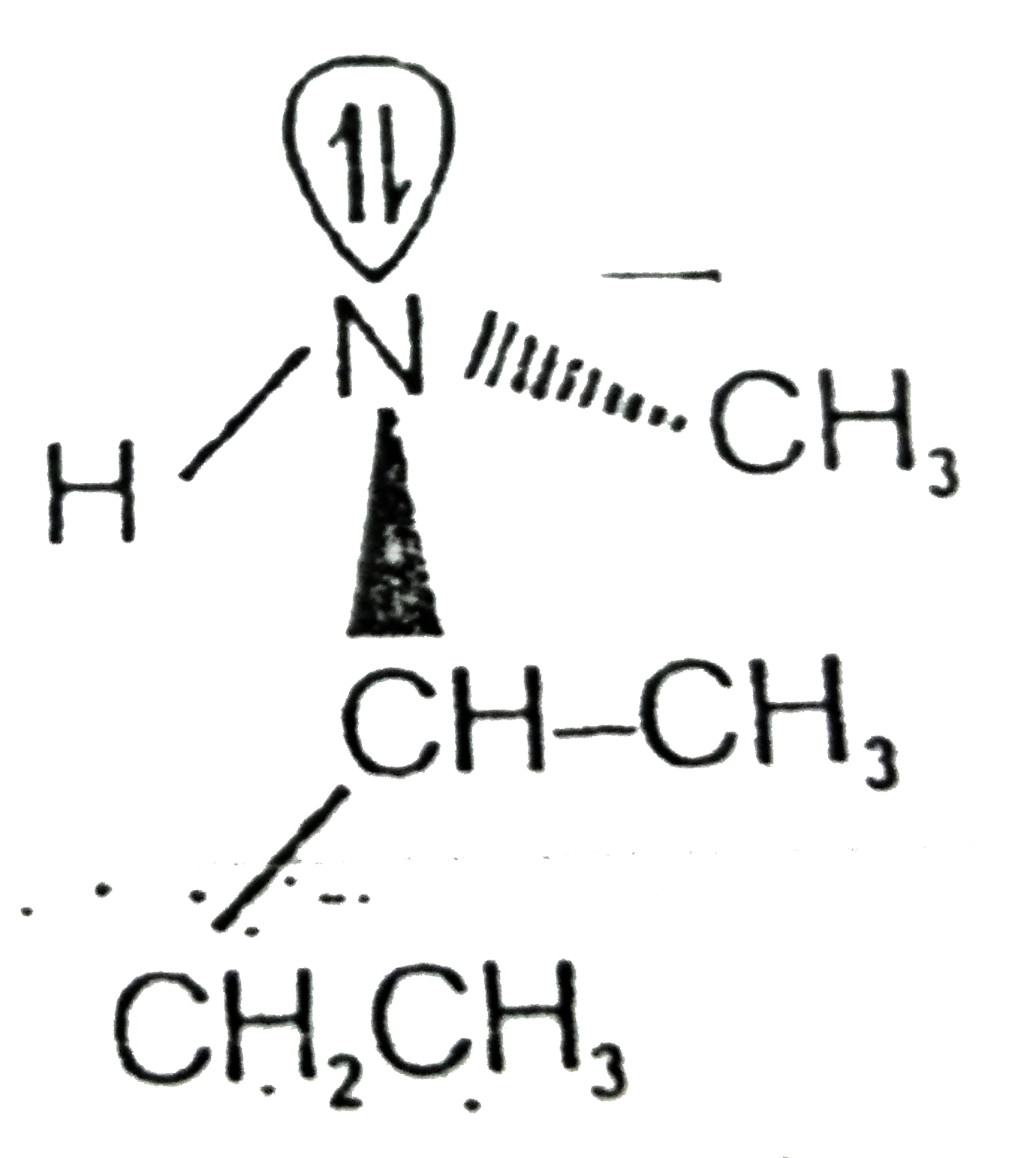A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - D) (Linked Comprehension Type Question )|8 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - E) ( Assertion - Reason type Questions )|7 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section - B ) ( Objective Type Question(One option is correct ))|36 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE ENGLISH|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HALOALKANES AND HALOARENES -Assignment (Section - C ) ( Objective Type Question(More than one options are correct ))
- Which of the following cannot be resolved into enantiomers ?
Text Solution
|
- Consider the following pair of compound which of the followin...
Text Solution
|
- Which of the following compound can be resolved into enatiomers ...
Text Solution
|
- Which of the following nitroso compound are in dynamic equilibrium ...
Text Solution
|
- Which of the following products are expected from the solvolysis ...
Text Solution
|
- What would be the probable products of the given reaction ?
Text Solution
|
- Under the presence of alkali which of the following substrate wil...
Text Solution
|
- Which of the following reactions follows concerted mechanism ?
Text Solution
|
- Dehydrohalogenation and acidcatalyzed dehydration reactions are fre...
Text Solution
|
- Which of the following alkylhalides will give one alkene (more t...
Text Solution
|
- When ethyl chloride reacts with ethanolic sodium nitrite produc...
Text Solution
|
- Which of the following reactions can be used to introduce bromin...
Text Solution
|
- Aryl halides are practically inert toward nucleophilic substitut...
Text Solution
|
- Consider the following reaction Correct statement regarding ...
Text Solution
|
- Which of the following statement are true about aryl halides ?
Text Solution
|
- Which of the following reagents can be used to convert alkyl ha...
Text Solution
|
- Consider the following sequence of reaction identify the str...
Text Solution
|