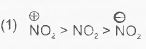
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-MOCK TEST 6-Example
- Octet rule is not satisfied for which of the following molecules?
Text Solution
|
- The ion that is isoelectronic with CO is
Text Solution
|
- The formal charge on S atom in the following structure is
Text Solution
|
- Elements of which of the following sets has the strongest tendency to ...
Text Solution
|
- Which of the following sequence correctly represents the decreasing ac...
Text Solution
|
- Element A has 3 electron in the outermost orbit and element B has 7 e...
Text Solution
|
- Compound of a metal A is M2O3 , the formula of its halide is
Text Solution
|
- As compared to covalent compounds, electrovalent compounds generally p...
Text Solution
|
- The magnitude of lattice energy of a solid increases if
Text Solution
|
- Which of the following is a hypervalent compound?
Text Solution
|
- Which of the following is a hypovalent compound?
Text Solution
|
- Which of the following is an odd electron molecule?
Text Solution
|
- The lattice energies of the oxides of Mg, Ca, Sr and Ba follow the ord...
Text Solution
|
- Which of the following is the correct order of bond angle in H2S, NH3,...
Text Solution
|
- Give the correct order of bond lengths x, y and z in
Text Solution
|
- Which of the following statements is/ are correct about lattice enthal...
Text Solution
|
- Find out the incorrect order of bond angles
Text Solution
|
- Anhydrous AlCl3 is covalent compound. Select the correct statement reg...
Text Solution
|
- The lattice enthalpy of KI will be, if the enthalpy of (I) Deltaf H^-...
Text Solution
|
- The species which do not support octet rule are (a) H2O (b) Cl2O (c)...
Text Solution
|