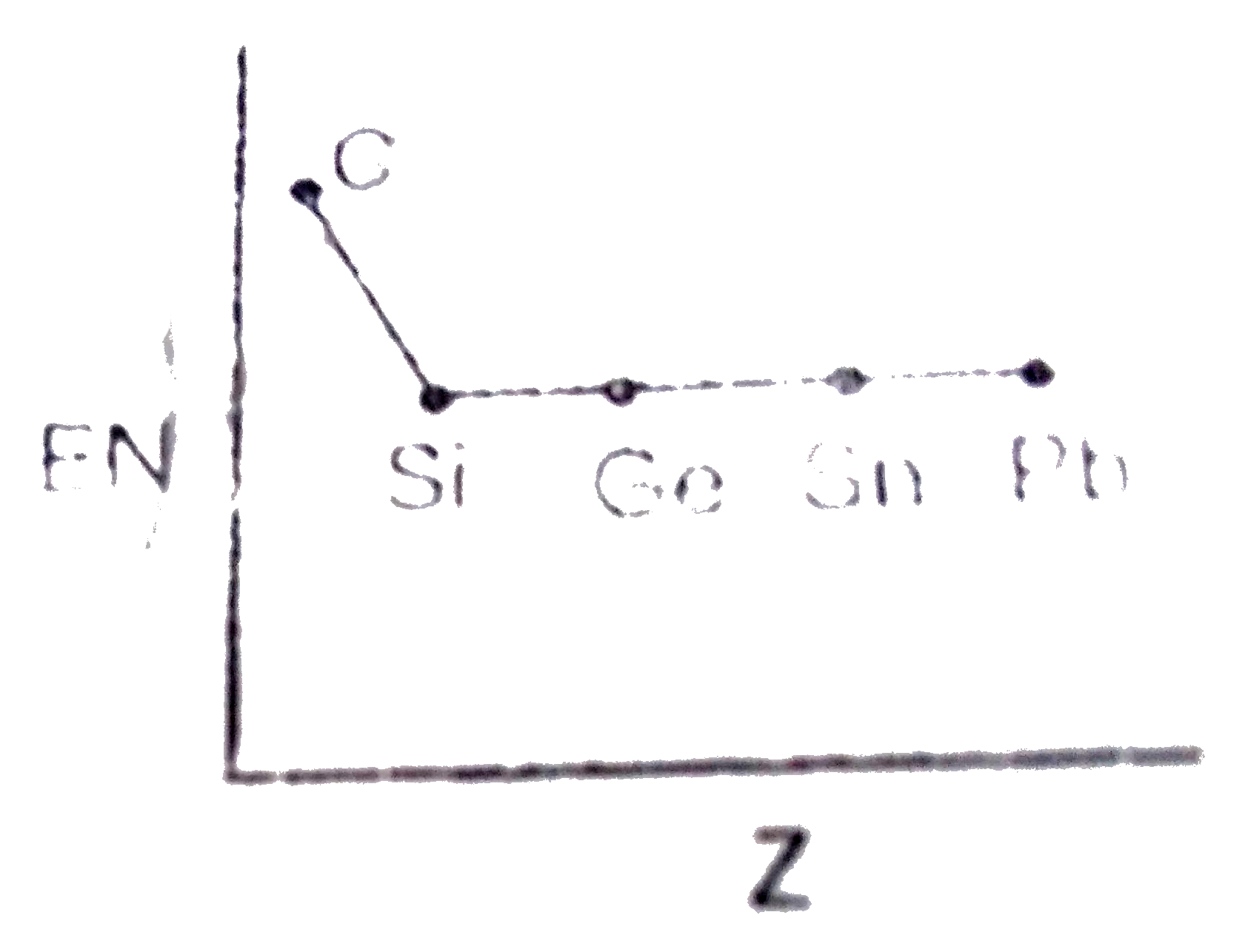A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise SECTION-1|7 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise SECTION-2|5 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise Exercise-3 Part-II : JEE (MAIN) / AIEE PROBLEMS (PREVIOUS YEARS)|12 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Advanced Level Problems Part-I : PRACTICE TEST-1 (IIT-JEE (MAIN Pattern))
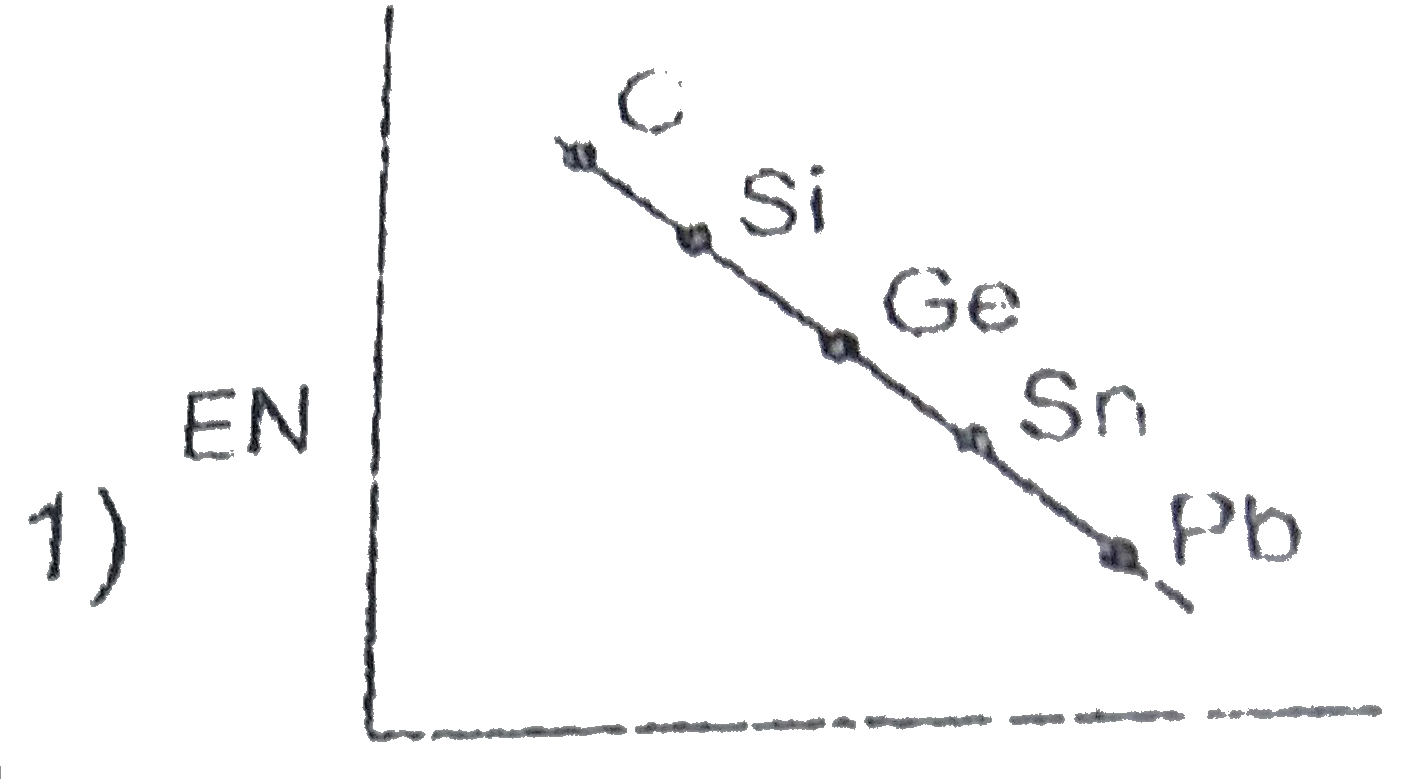
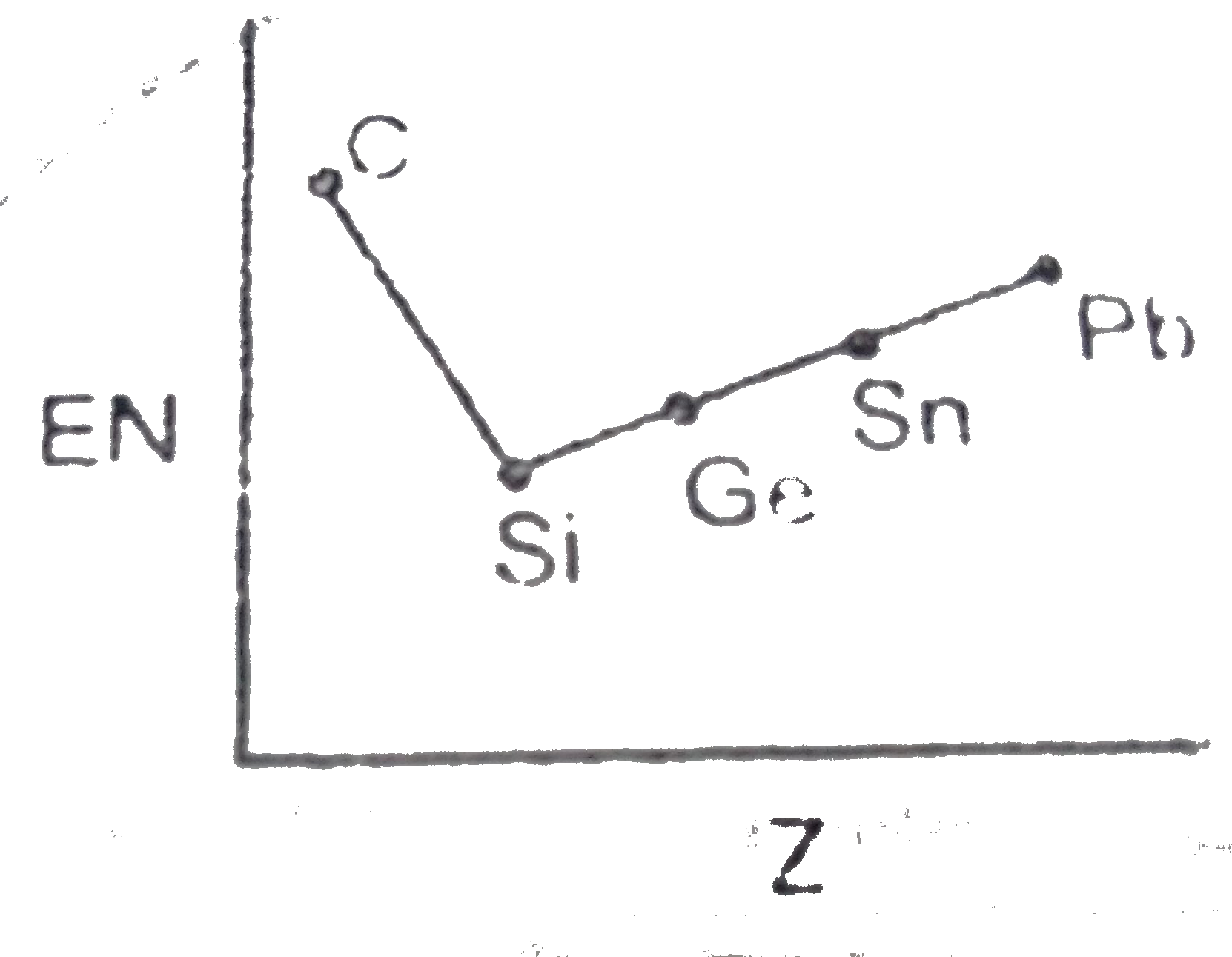
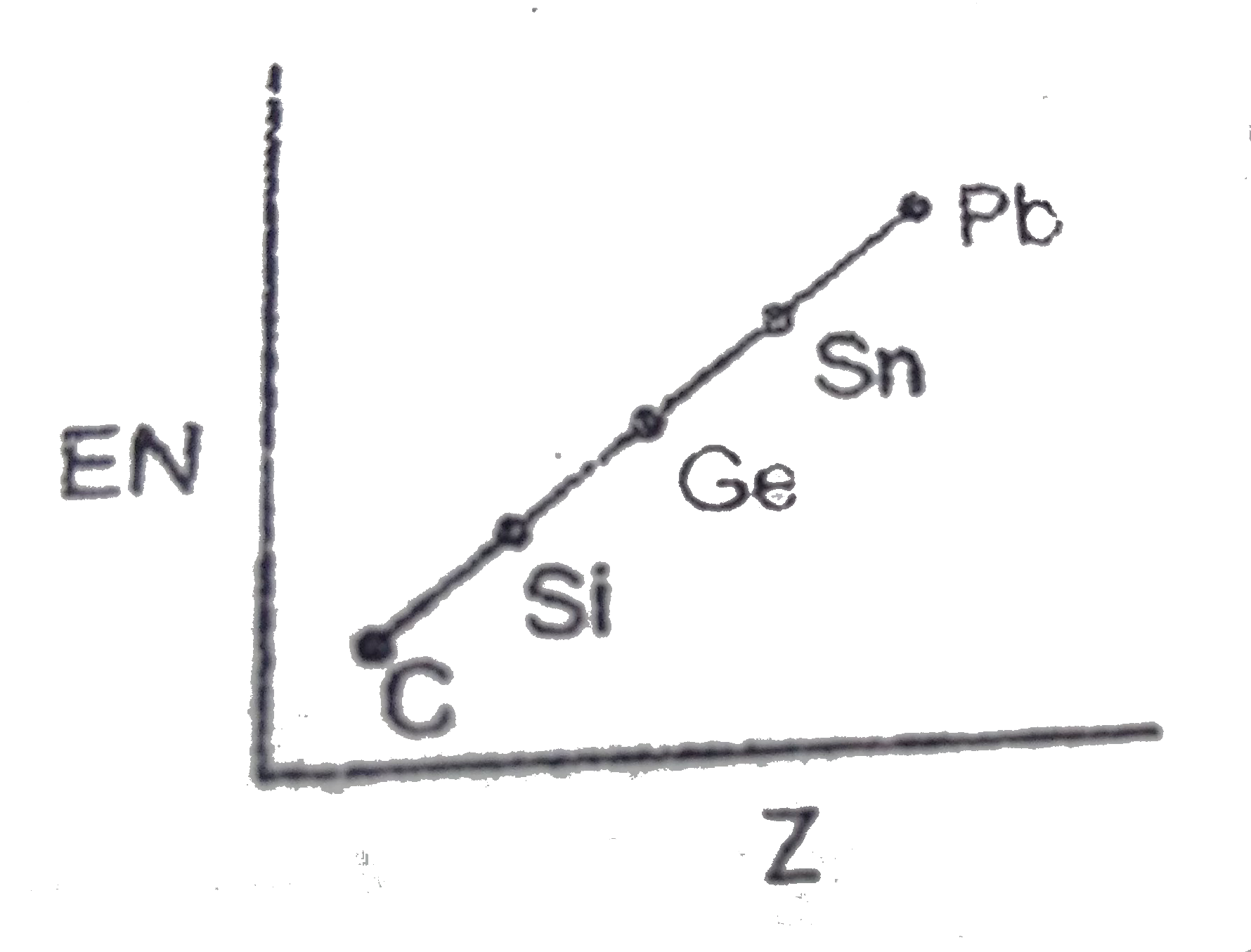
- Which of the following is the correct graph for EN values of carbon fa...
Text Solution
|
- Select the incorrect statement :
Text Solution
|
- Which of the following statement about Si is correct.
Text Solution
|
- Identify correct statement.
Text Solution
|
- 4BCl(3)+ 3LiAIH(4) to A+ 3AlCl(3)+3 LiCl When A reacts with NaOH it...
Text Solution
|
- When heating white lead then find out released gas (A) and (B) under...
Text Solution
|
- Amorphous boron is extracted from borax by the following steps : "Bo...
Text Solution
|
- The role of addition of Me(3)SiCl during the hydrolysis followed by co...
Text Solution
|
- Given type of silicones are called [P] -O-oversetoverset(R)(|)unders...
Text Solution
|
- Which of the following is not a property of silicones ?
Text Solution
|
- Tourmalene is a class of cyclosilicates with general formula. (Ca, K...
Text Solution
|
- The silicate anion in the mineral kinoite is a chain of three SiO(4) t...
Text Solution
|
- The dehydration of malonic acid CH(2) (COOH)(2) with P(4)O(10) and hea...
Text Solution
|
- Borax on heating with cobalt oxide forms a blue bead of
Text Solution
|
- The dissolution of Al(OH)(3) by a solution of NaOH results in the form...
Text Solution
|
- Select the incorrect statement about the boron.
Text Solution
|
- Aqueous solution containing 1 mol of borax raects with 2 mol of acids....
Text Solution
|
- Water transpoted through Lead pipes becomes poisonous due to the forma...
Text Solution
|
- When a mixture of air and steam is passed over red hot coke, the outgo...
Text Solution
|
- In BF(3), the B-F bond length is 1.30 A, when BF(3) is allowed to be t...
Text Solution
|