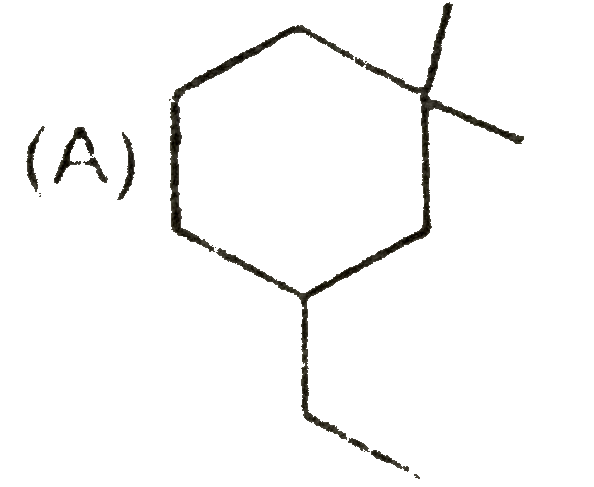
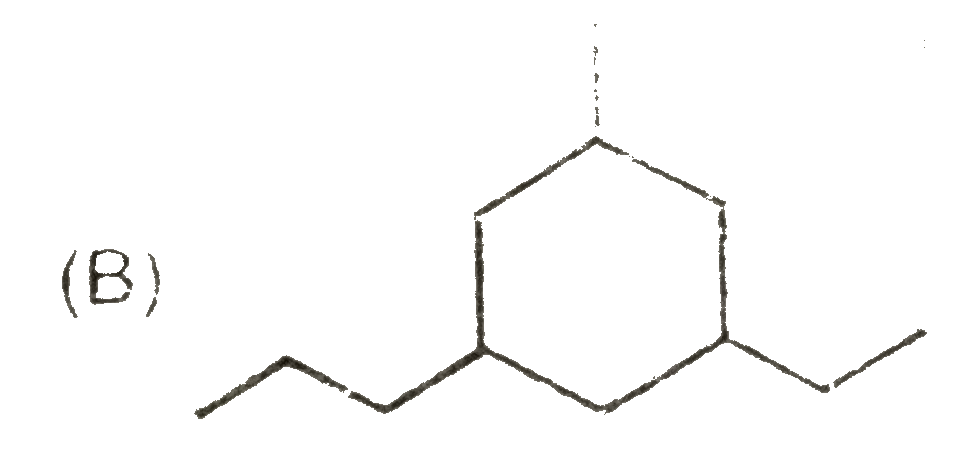
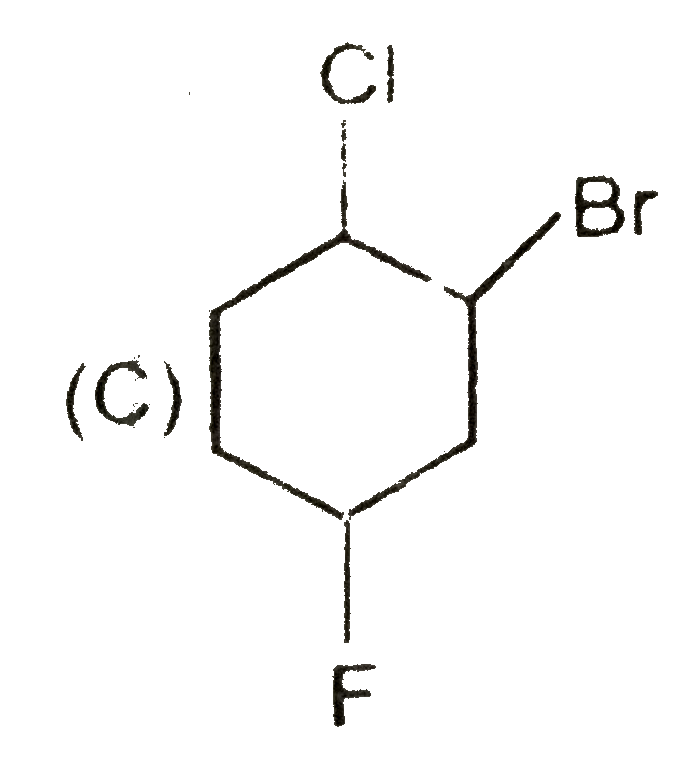
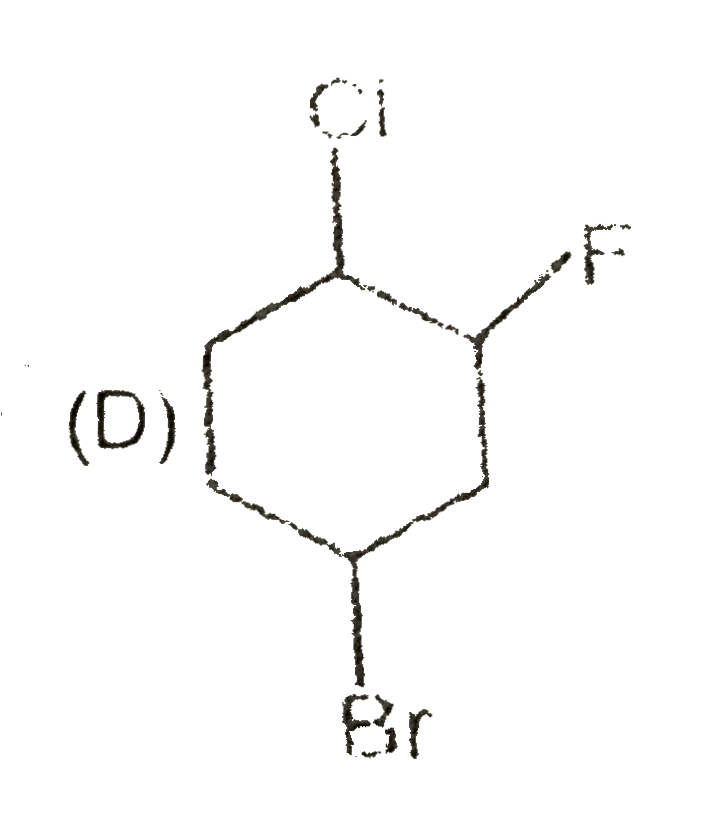
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-2 Part-4|2 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-3 Part-1|9 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-2 Part-2|12 VideosIONIC EQUILIBRIUM
RESONANCE ENGLISH|Exercise partIII one or more than one options correct type|10 VideosMETALLURGY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|17 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM -Exercise-2 Part-3
- All the members of a homologus series have same
Text Solution
|
- The pair of compounds having the same general formula.
Text Solution
|
- Which of the following IUPAC names are correct.
Text Solution
|
- The compound with only primary hydrogen atoms is/are:
Text Solution
|
- Which of the following is/are incorrect IUPAC name/ (s):
Text Solution
|
- What is Double bond equivalent ? Explain with formula .
Text Solution
|
- Which of the following IUPAC name is incorrect ?
Text Solution
|
- Which of the following is the correct relationship?
Text Solution
|
- Which of the following are functional isomers of methyl ethanoate?
Text Solution
|
- Which of the following can be the isomer(s) of C(8)H(8)O:
Text Solution
|