A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-2 Part-2: Single and double value integer type|14 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-2 Part-3: One or more than type one options correct type|11 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-1 Part-I Subjective question|30 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise-2 Part-1: Only one option correct type
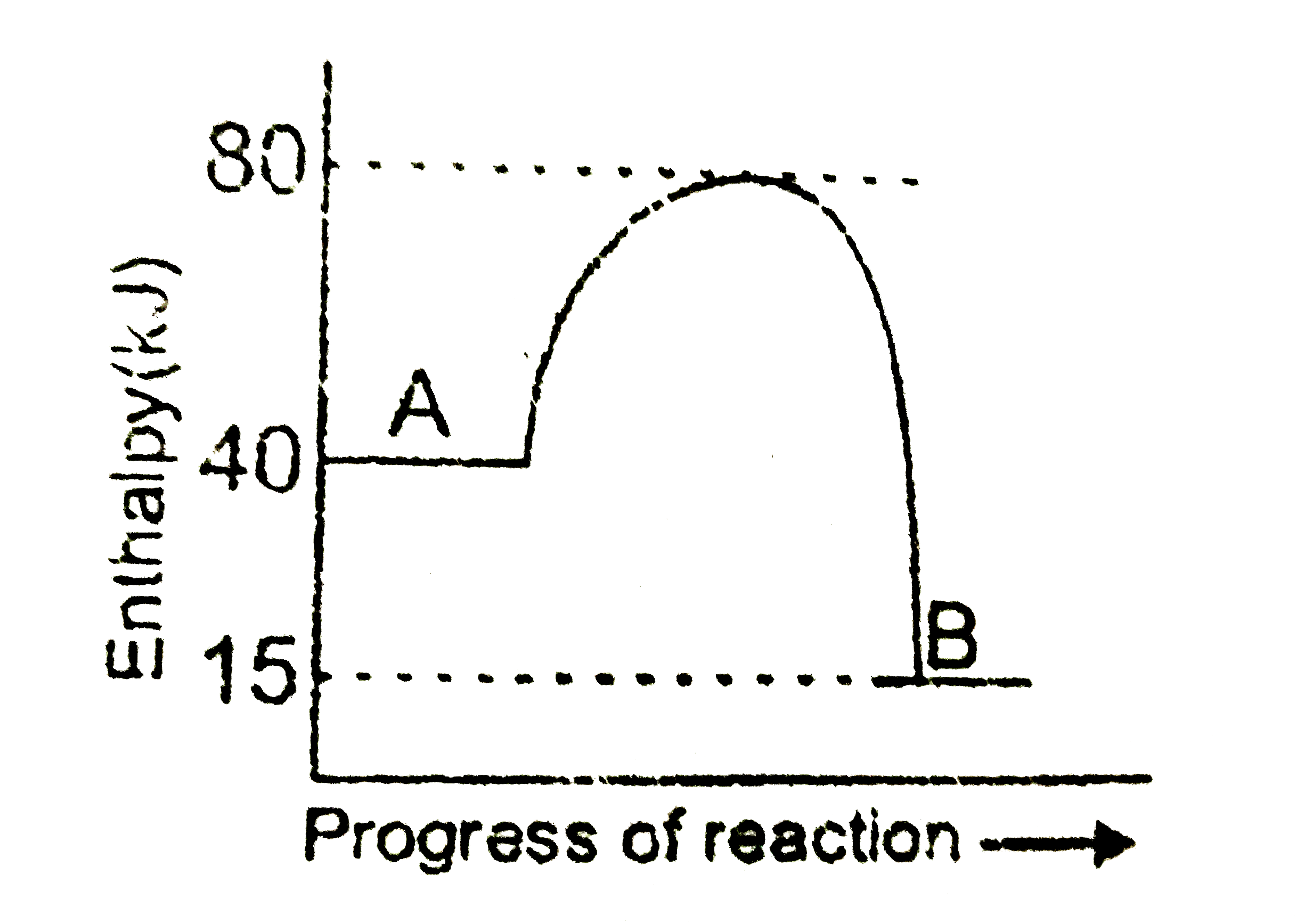
- Look at the following diagram: The enthalpy change for the reacti...
Text Solution
|
- The difference between Delta H and Delta E (on a molar basis) for the ...
Text Solution
|
- The heat of formation of HCl at 348 K from the following data will be ...
Text Solution
|
- In the Haber 's process of ammonia manufacture: N(2) (g)+3H(2) (g) t...
Text Solution
|
- From the following data of Deltah, of the following reactions, a. C(...
Text Solution
|
- When 12.0g of C reacted with a limited quantity of oxygen, 57.5 kcal o...
Text Solution
|
- The standerd enthalpy of formation of FeO and Fe(2)O(3) is -65 kcal "m...
Text Solution
|
- In the reaction AB(2)(l)+3X(2)(g)hArrAX(2)(g)+2BX(2)(g)+2BX(2)(g)Delta...
Text Solution
|
- Reactions involving gold have been of particular intrests to alchemist...
Text Solution
|
- The heat of formation of C(2)H(5)OH(l) is -66 kcal//"mole". The heat o...
Text Solution
|
- Calculate the enthalpy change for the following reaction: XeF(4) rar...
Text Solution
|
- Caesium chlorides is formed according to the following equation Cs(s)+...
Text Solution
|
- The enthalpies of neutralization of a weak base AOH and a strong base ...
Text Solution
|
- Equal volumes of molar hydrochloric acid and sulphuric acid are neutra...
Text Solution
|
- The enthalpy of neutralization of 40.0 g of NaOH by 60.0 g of CH(3)COO...
Text Solution
|
- Given Delta(i)H^(Theta)(HCN) = 45.2 kJ mol^(-1) and Delta(i)H^(Theta)(...
Text Solution
|
- A solution is 500 ml of 2M KOH is added to 500 ml of 2 M HCl and the m...
Text Solution
|
- 50.0 mL of 0.10 M HCl is mixed with 50.0 mL of 0.10 M NaOH. The soluti...
Text Solution
|
- The average O-H bond energy in H(2)O with the help of following data. ...
Text Solution
|
- The average energy required to break a P-P bond in P(4)(s) into gaseou...
Text Solution
|