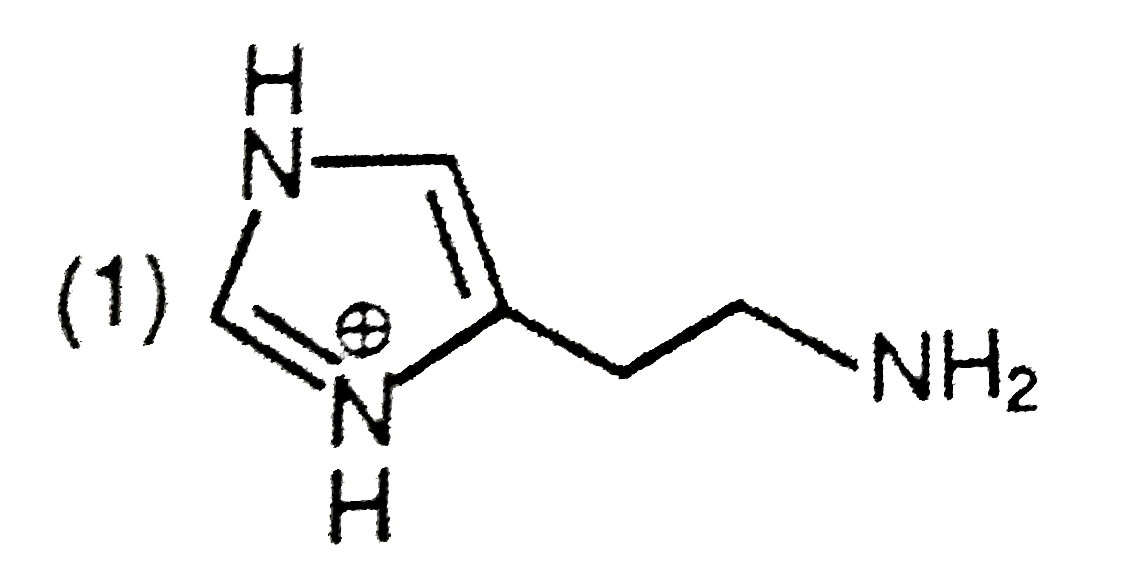
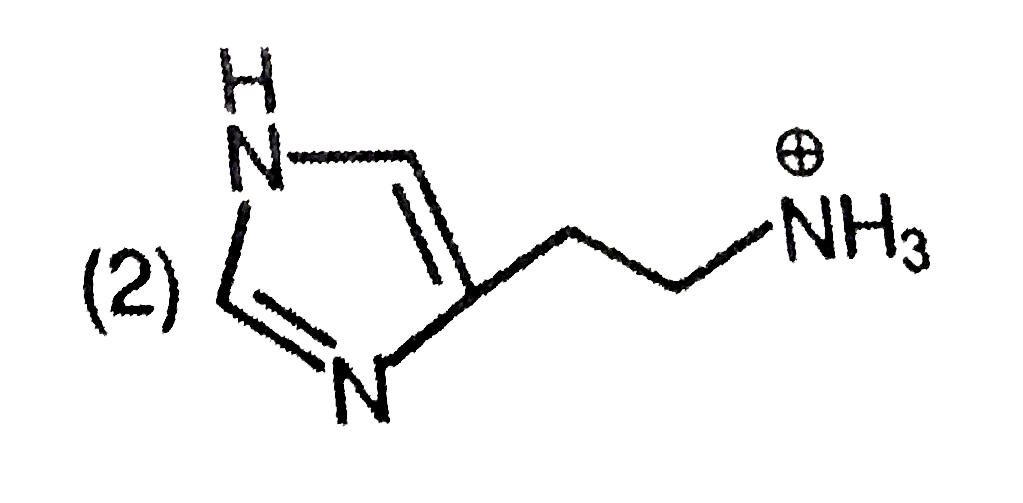
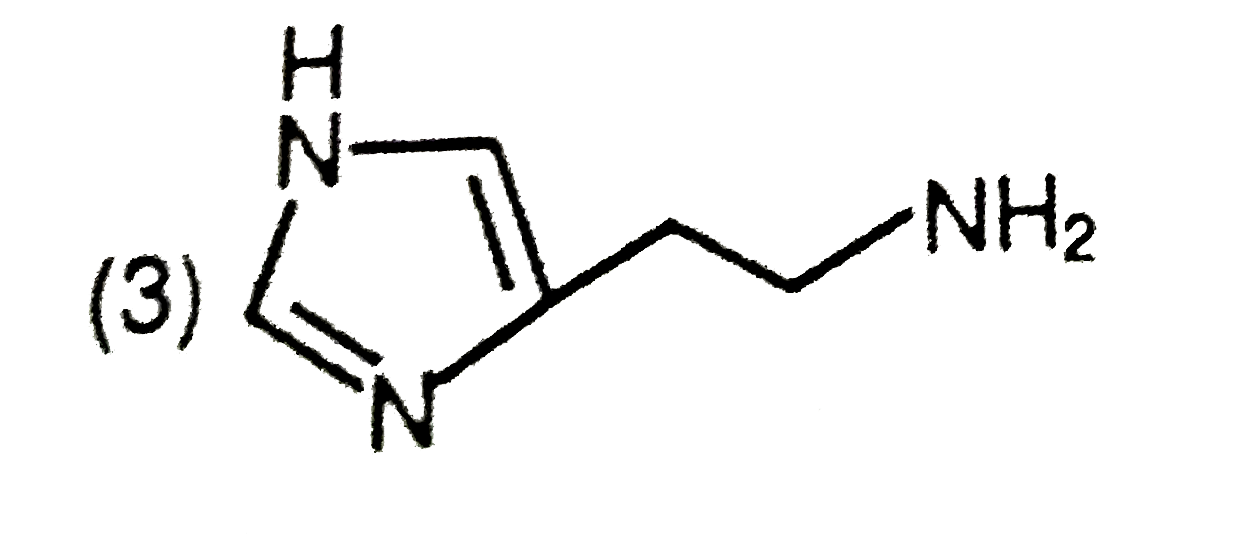
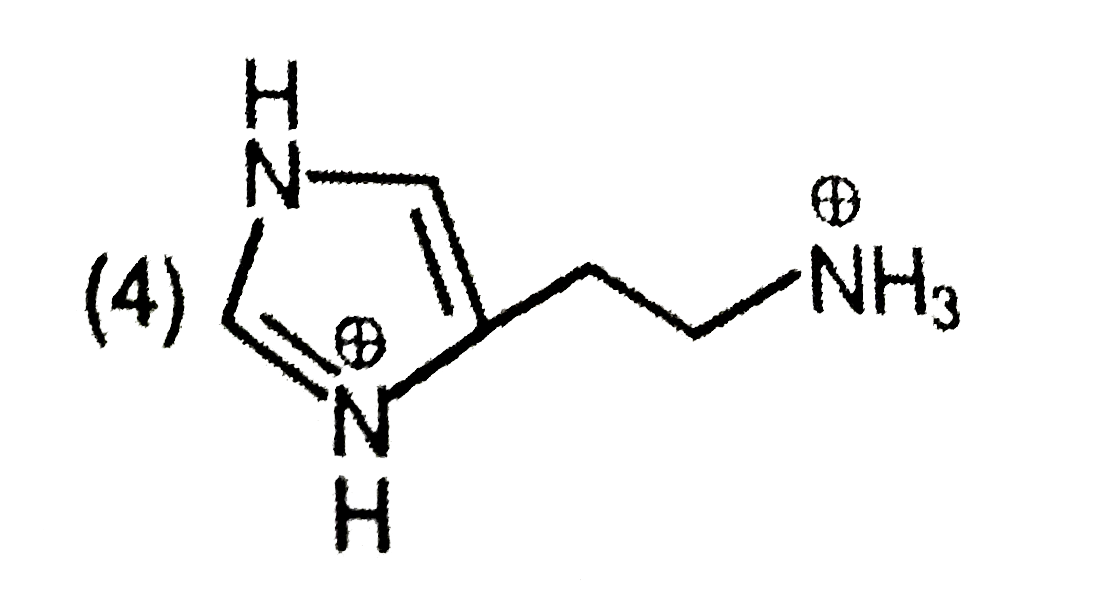
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS|16 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise (APSP) Part-I:Practice Test-1|30 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:I JEE(Advanced)/IIT-JEE Problems (Previous Years)|17 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)
- The correct order of increasing basic nature for the bases NH3....
Text Solution
|
- Which of the following is the strongest base ?
Text Solution
|
- Consider the acidity of the carboxylic acids : (i) PHCOOH" "(ii) ...
Text Solution
|
- Among the following acids which has the lowest pk(a) value-
Text Solution
|
- Amongest the following the most basic compounds is-
Text Solution
|
- The increasing order of stability of the following free radicals is
Text Solution
|
- The correct order of increasing acid strength of the compounds is (a...
Text Solution
|
- Which one of the following is the strongest base in aqueous solution ?
Text Solution
|
- Arange the carbanions, (CH(3))(3) overset(bar)(C), overset(bar)(C)Cl(3...
Text Solution
|
- The correct order of increasing basicity of the given conjugate bases ...
Text Solution
|
- The strongest acid amongst the following compounds is?
Text Solution
|
- Identify the compound that exhibits tautomerism: 2-butene lactic...
Text Solution
|
- The correct order of acid strength of the following compounds:
Text Solution
|
- Arrange the following in decreasing order of acidic nature of
Text Solution
|
- The order of stability of the following carbocations:
Text Solution
|
- Considering the basic strength of amines in aqueous solution, which on...
Text Solution
|
- The increasing order of basicity of the following compounds is
Text Solution
|
- The predominant form of histamine present in human blood is (pK(a) , H...
Text Solution
|