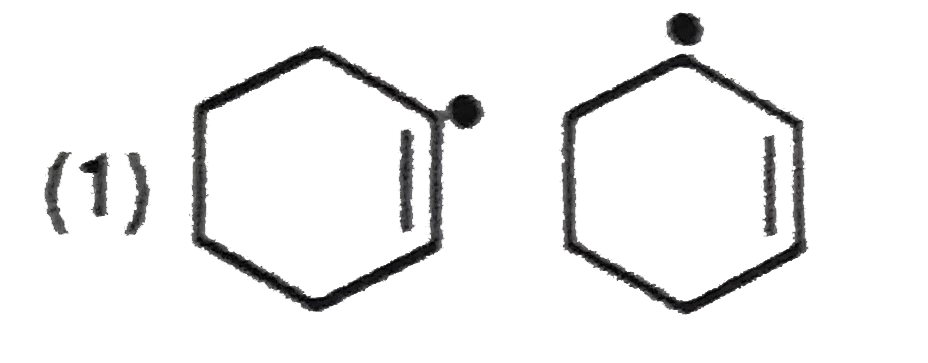
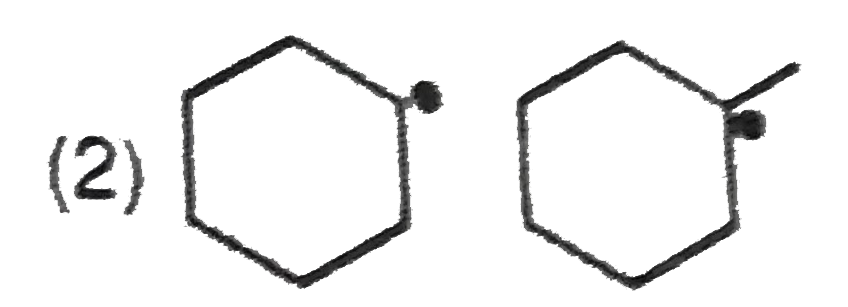
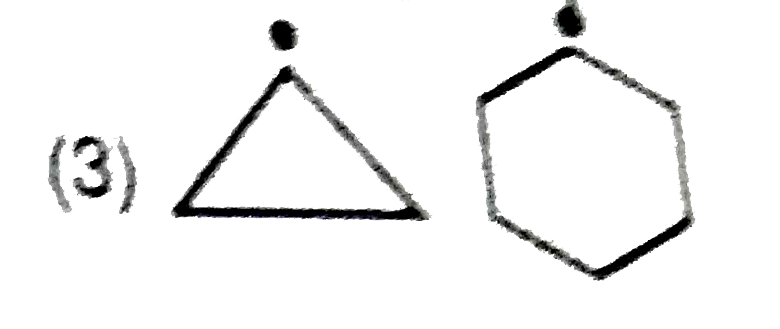
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise (APSP) Part-I:Practice Test-1|30 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-II: NSEC (Stage-I)|45 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)|18 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS
- In which of the following pairs A is more stable than B?
Text Solution
|
- Which one of the following statements is not correct ?
Text Solution
|
- Which one of the following substituents at para-position is most effec...
Text Solution
|
- Which of the following will not be soluble in sodium hydrogen carbonat...
Text Solution
|
- Arrange the following amines in the order of increasing basicity :-
Text Solution
|
- The ''N'' which contribute least to the basicity for the compound is :
Text Solution
|
- Among the following compounds, the increasing order of their basic str...
Text Solution
|
- The inceasing order of the acidity of the following carboxylic acids i...
Text Solution
|
- Which amongst the following is the strongest acid ?
Text Solution
|
- The correct decreasing order for acid strength is :
Text Solution
|
- Arrange the follwing amines in the decreasing order of basicity :
Text Solution
|
- The increasing basicity order of the following compounds is: (A) CH(...
Text Solution
|
- The increasing order of the pKa values of the following compounds is
Text Solution
|
- Which of the following compounds will produce a precipitate with aGno(...
Text Solution
|
- In the following compound, The favourable site/s for protonation ...
Text Solution
|
- The correct order for acid strength of compounds CH-=CH,CH(3)-C-=CH ...
Text Solution
|