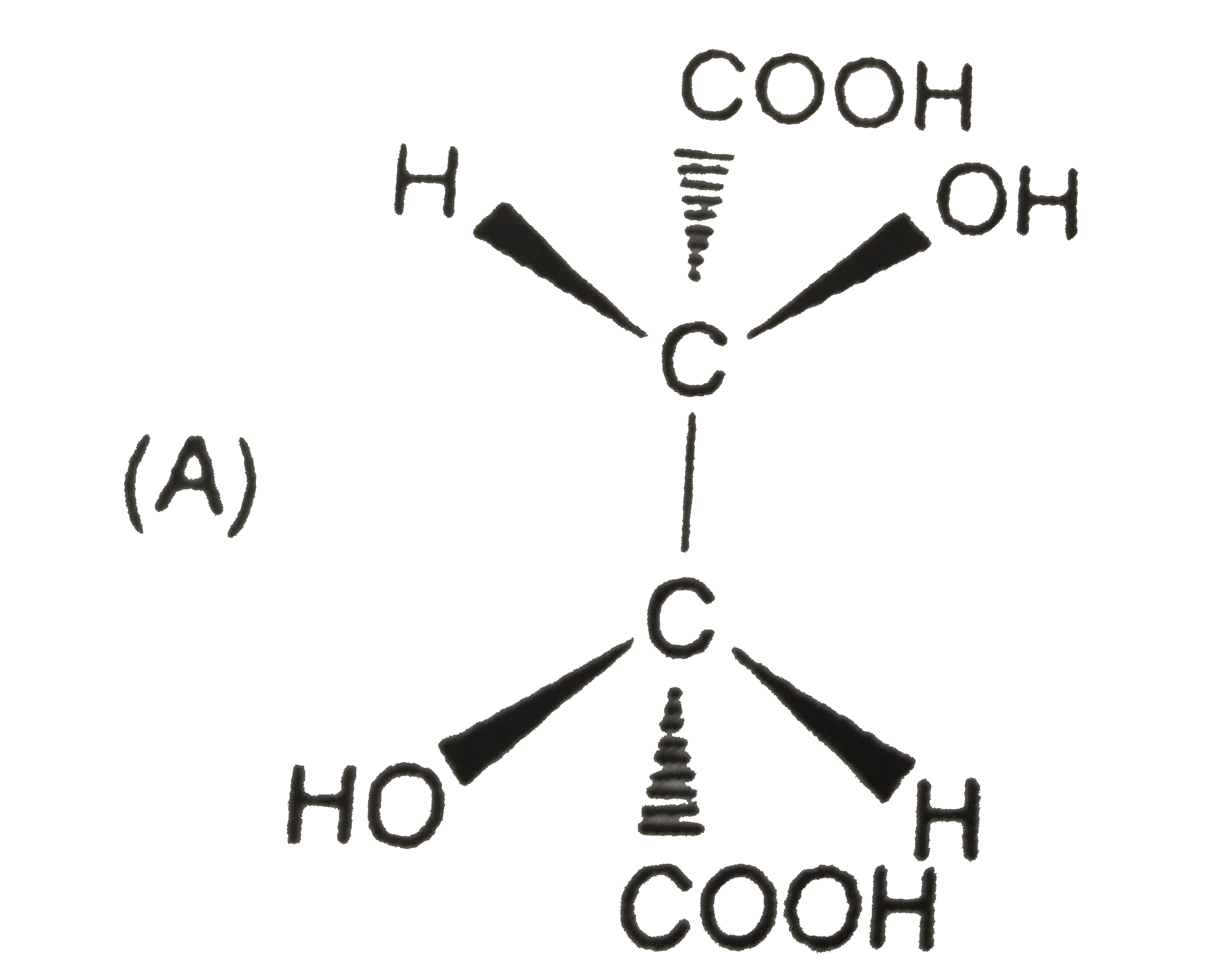
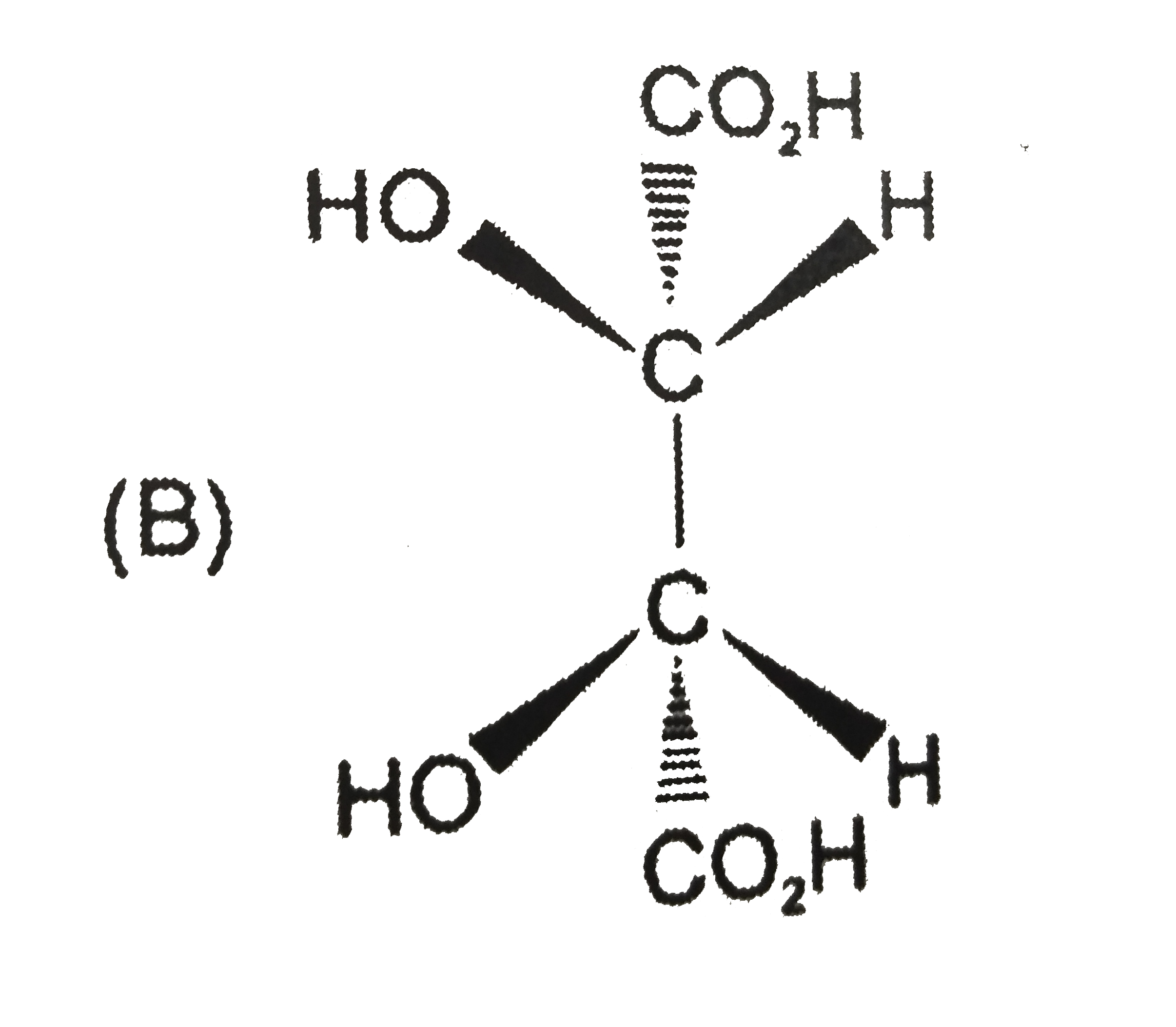
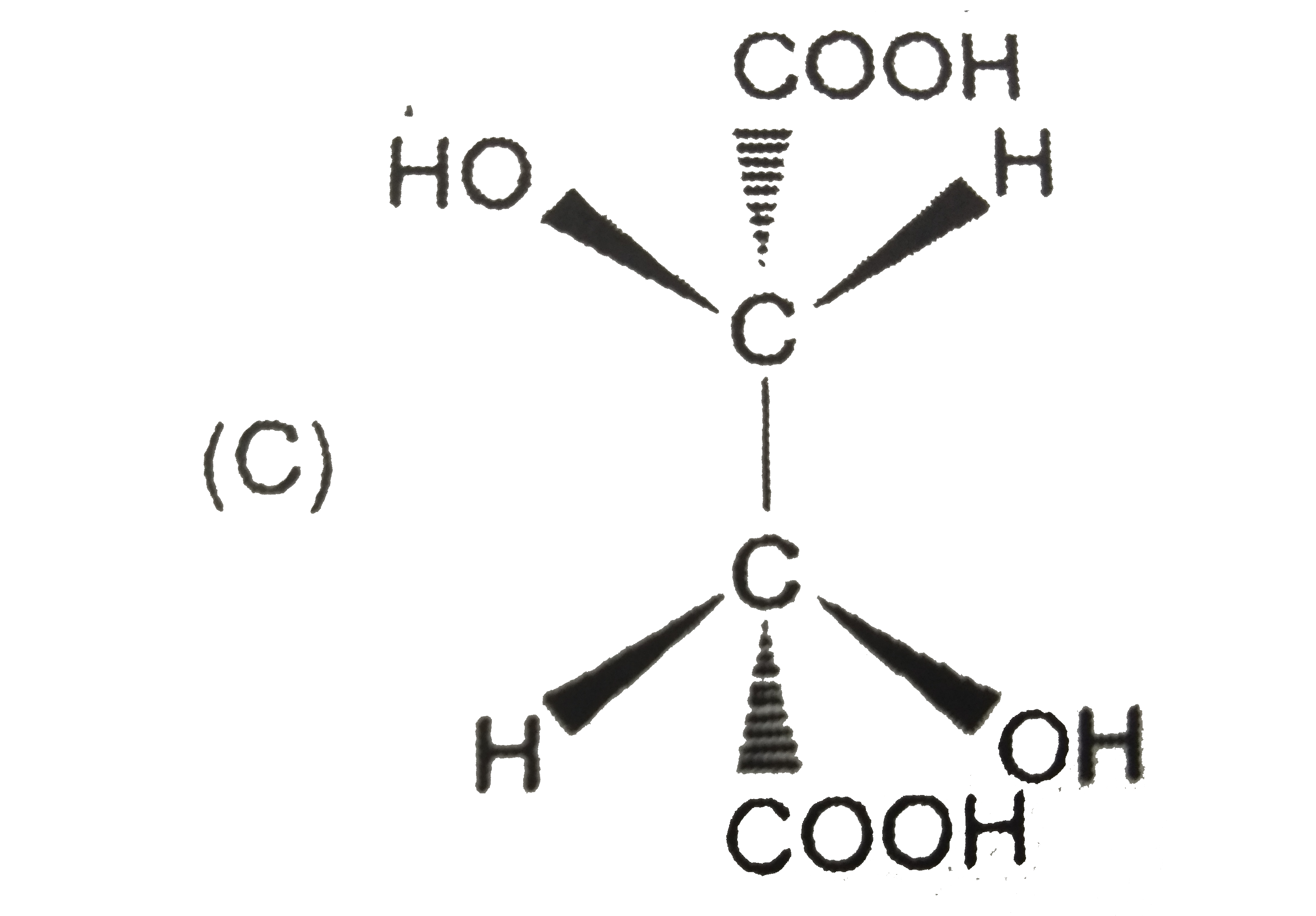A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART -1 JEE (ADVANCED))|16 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART -II JEE (MAIN))|16 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE)|11 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (PART IV : COMPREHENSION)
- Tartaric acid [HO(2)CCH(OH)CH(OH)CO(2)H] was an important compound in ...
Text Solution
|
- Tartaric acid [HO(2)CCH(OH)CH(OH)CO(2)H] was an important compound in ...
Text Solution
|
- The only correct combination is -
Text Solution
|
- Appropriately match the information given in the three columns of the ...
Text Solution
|
- appropriately matching the information given in the three columns of t...
Text Solution
|