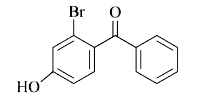
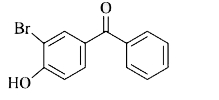
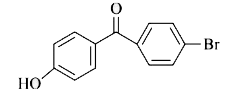
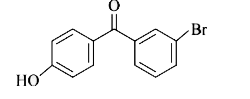
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST 11 (2020) -CHEMISTRY (SECTION - 2)
- p-Hydroxybenzophenone upon reaction with bromine in carbon tetrachlori...
Text Solution
|
- What would be the molality of 60% (mass/mass) aqueous solution of CH(3...
Text Solution
|
- During compression of a spring the work done is 10 kJ and 2 kJ escaped...
Text Solution
|
- Molal depression constant for a solvent is 4.0 K kg mol^(-1). The de...
Text Solution
|
- Molecules from 10mL of 1mM surfactant solution are adsorbed on 0.24cm^...
Text Solution
|
- The maximum possible denticities of a ligand given below towards inner...
Text Solution
|