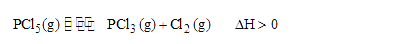A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST -17 (2020)-CHEMISTRY
- The gas phase reaction PCl(5)(g)<=> PCl(3)(g)+Cl(2)(g) is an endother...
Text Solution
|
- Which one of the following has triangleS^(@) positive?
Text Solution
|
- Which of the following is not a correct statement?
Text Solution
|
- The kinetic energy of the electron in the second Bohr's orbit of a hyd...
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Among the following compounds of phosphorus: H(4)P(2)O(6), P(2)O(5),...
Text Solution
|
- A metal complex having composition has been isolated in two forms (X)...
Text Solution
|
- Complex formed in the following methods are (I) Mond's process for p...
Text Solution
|
- Which of the following conversions involves change in both shape and h...
Text Solution
|
- Which of the following order is incorrect ?
Text Solution
|
- FeCr(2)O(4)( chromite ) is converted to Cr by following steps : Chro...
Text Solution
|
- Identify the major product A. CH(3)-CH=CH-CH=CH(2) underset((40^(@)C))...
Text Solution
|
- The barrier for rotation about indicated bonds will be maximum in whic...
Text Solution
|
- Which of the following is incorrect?
Text Solution
|
- Barbituric acid and its derivatives are well known as
Text Solution
|
- Select the final product of the following sequence of reaction
Text Solution
|
- Identify Y:
Text Solution
|
- KI in Acetone undergoes SN2 reaction with each of P, Q, R, S. The dec...
Text Solution
|
- Sodium phenoxide when heated with CO(2) under pressure at 125^(@) C y...
Text Solution
|