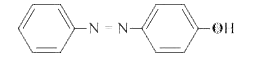
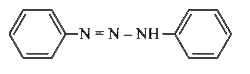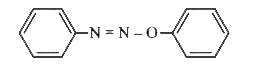A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 12 -CHEMISTRY
- Aniline dissolved in dilute HCL is reacted with sodium nitrate at 0^(@...
Text Solution
|
- Which of the following are path functions (A) W (B) Q (C) Q+W ...
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- Liquid ‘M’ and liquid ‘N’ form an ideal solution. The vapour pressures...
Text Solution
|
- The organic compound that gives following qualitative analysis is : ...
Text Solution
|
- The correct IUPAC name of the following compound is:
Text Solution
|
- Excessive release of CO(2) into the atmosphere result in :
Text Solution
|
- Which of the following statements is not true about sucrose?
Text Solution
|
- major product of the following reaction is : CH(3)CH=CHCO(2)CH(3) over...
Text Solution
|
- The degenerate orbitals of [Cr(H(2)O)(6)]^(3+) are:
Text Solution
|
- The major product of the following reaction is CH(3)C-=CH underset((ii...
Text Solution
|
- The increasing order of reactivity of the following compounds towards ...
Text Solution
|
- The one that will show optical activity is: (en = ethane-1,2-diamine)
Text Solution
|
- C(60) , an allotrope of carbon contains :
Text Solution
|
- Match the catalysts (column I) with products (Column II)
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- Consider the van der Waals constants, a and b, for the following gases...
Text Solution
|
- The aerosol is a kind of colloid in which :
Text Solution
|
- The correct order of the oxidation states of nitrogen in NO, N(2)O, N...
Text Solution
|
- Among the following, the molecule expected to be stabilized by anion f...
Text Solution
|