Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 10| JEE -2020-CHEMISTRY (SECTION 2)
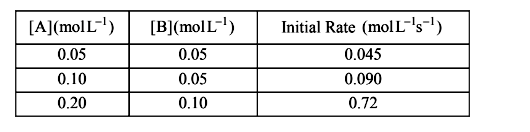
- For the reaction 2A+BrarrC, the values of initial rate at different r...
Text Solution
|
- In order to oxidise a mixture of one mole of each of FeC(2)O(4).Fe(2)(...
Text Solution
|
- 100mL of a water sample contains 0.81g of calcium bicarbonate and 0.73...
Text Solution
|
- Coupling of benzene diazonium chloride with 1-naphthol in alkaline med...
Text Solution
|
- What is maximum denticity of the following ligand ?
Text Solution
|