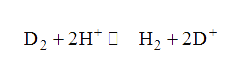Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 27 - JEE -2020-CHEMISTRY (SECTION 2)
- A solution containing H^(+) and D^(+) ions is in equilibrium with a mi...
Text Solution
|
- A gas (C(v.m)=(5)/(2)R) behaving ideally was allowed to expand reve...
Text Solution
|
- 2.0 g of a sample contains mixture of SiO(2) and Fe(2)O(3). On ver...
Text Solution
|
- Find the total number of monohalogenated products including stereoisom...
Text Solution
|
- Calculate value of (X+Y+Z)/(10), here X is O-N-O bond angle in NO(3)^(...
Text Solution
|