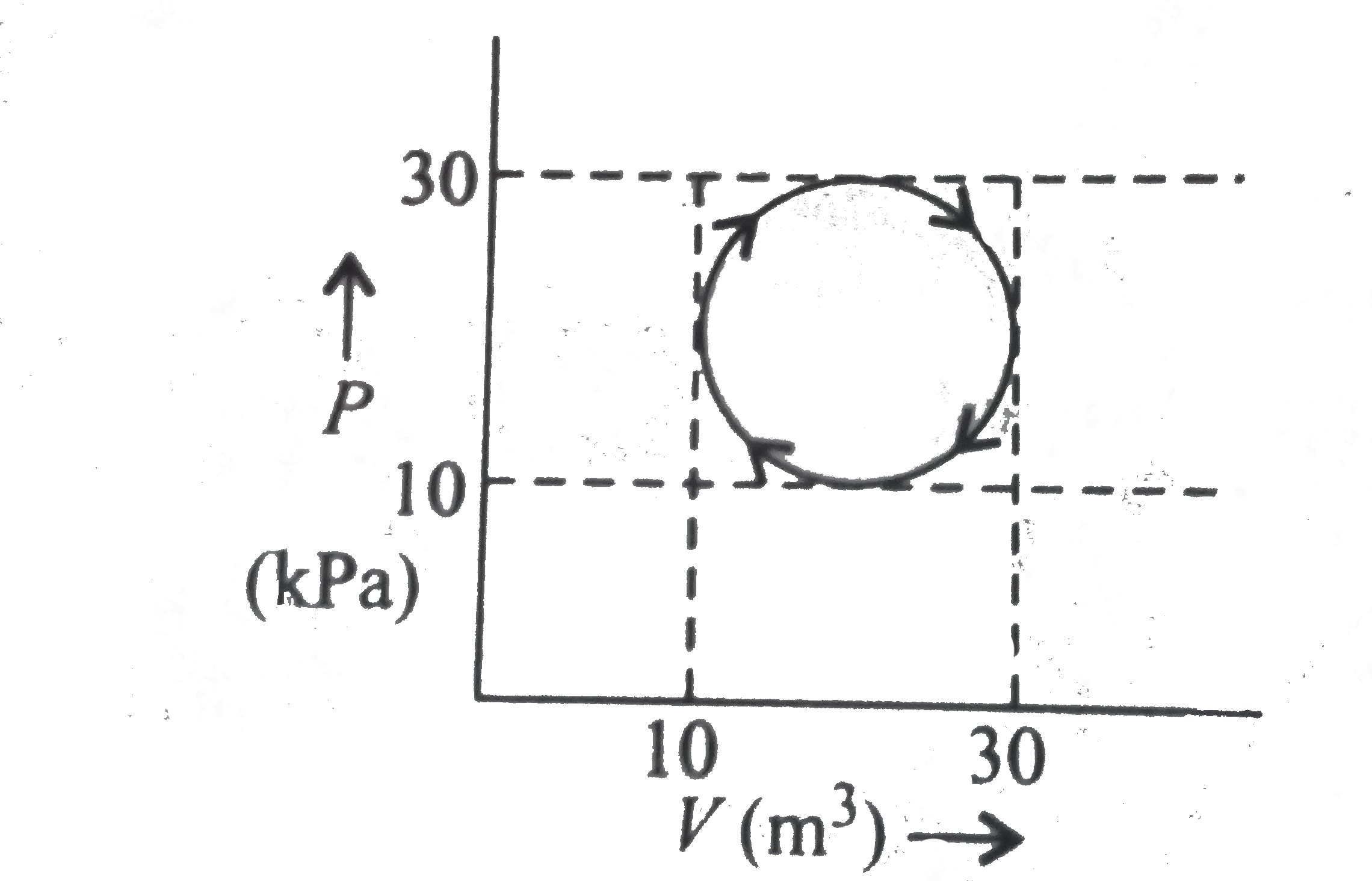
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CAPACITORS
VMC MODULES ENGLISH|Exercise JEE Advance ( Archive ) LEVEL 16|1 VideosCAPACITORS
VMC MODULES ENGLISH|Exercise JEE Advance ( Archive ) LEVEL 17|1 VideosCAPACITORS
VMC MODULES ENGLISH|Exercise JEE Advance ( Archive ) LEVEL 14|1 VideosBASIC MATHEMATICS & VECTORS
VMC MODULES ENGLISH|Exercise Impeccable|50 VideosCURRENT ELECTRICITY
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|10 Videos
Similar Questions
Explore conceptually related problems