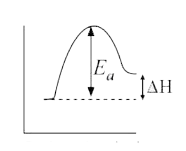
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|44 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-JEE (MAIN ARCHIVE)
- For an endothermic reaction, Delta H represents the enthalpy of the re...
Text Solution
|
- Identify the intensive quantity from the following
Text Solution
|
- Which of the following is an endothermic reaction?
Text Solution
|
- A process is called reversible when
Text Solution
|
- Which one of the following statements is false?
Text Solution
|
- Two moles of an ideal gas expanded isothermally and reversibly from 1L...
Text Solution
|
- Which of the following statements is/are false?
Text Solution
|
- Among the following, the intensive property is (properties are):
Text Solution
|
- A piston filled with 0.04 mol of an ideal gas expands reversibly from ...
Text Solution
|
- The standard free energy of formation of NO(g) is 86.6 kJ/ mol at 29...
Text Solution
|
- DeltaU is equal to
Text Solution
|
- The combination of plots which does not represent isothermal expansion...
Text Solution
|
- Consider the reversible isothermal expansion of an ideal gas in a clos...
Text Solution
|
- The entropy change associated with the conversion of 1 kg of ice at 27...
Text Solution
|
- An ideal gas undergoes isothermal compression from 5 m^(3) to 1 m^(3) ...
Text Solution
|
- The process with negative entropy change is
Text Solution
|
- Two bolcks to the same metal having same mass and at temperature T1 an...
Text Solution
|
- for a diatomic ideal gas in a closed system , which of the follo...
Text Solution
|
- A process has Delta H= 200 J mol^(-1) and DeltaS=40" JK"^(-1)mol^(-1)....
Text Solution
|
- The standard reaction Gibbs energy for a temperature T is given by ...
Text Solution
|