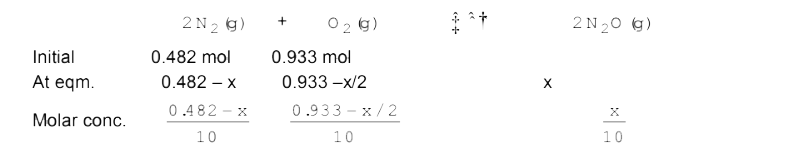Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 LA|6 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 1|75 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 SA I|7 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 0 SA II
- Reaction between N(2) and O(2-) takes place as follows : 2N(2)(g) + ...
Text Solution
|
- A mixture of 1.57 mol of N(2), 1.92 mol of H(2) and 8.13 mol of NH(3)...
Text Solution
|
- Predict which of the following reaction will have appreciable concentr...
Text Solution
|
- At 1,000 K in the reaction CO(2) (g) + C(s) rarr 2CO(g) The value of P...
Text Solution
|
- A mixture of 1.57 mol of N(2), 1.92 mol of H(2) and 8.13 mol of NH(3)...
Text Solution
|
- The value of K(c) for the reaction 2A rarr B + C "is" 2 xx 10^(-2) At ...
Text Solution
|