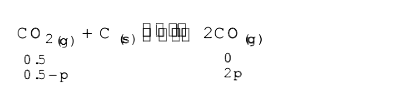A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 2|65 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|24 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise Level 0 LA|6 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Level 1
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- Four moles of PCl(5) are heated in a closed 4 dm^(3) container to reac...
Text Solution
|
- Consider the following two gaseous equilibria involving SO2 and the co...
Text Solution
|
- NH(4)HS(s) hArr NH(3)(g)+H(2)S(g) In the above reaction, if the pres...
Text Solution
|
- 3 moles of A and 4 moles of B are mixed together and allowed to come i...
Text Solution
|
- Which of the following is a wrong statement about equilibrium state ?
Text Solution
|
- A+B rarr C+D Initially moles of A and B are equal. At equilibrium, mol...
Text Solution
|
- Which of the following is not a physical equilibrium ?
Text Solution
|
- 2HI(g) rarr H(2)(g) + I(2)(g) The equilibrium constant of the above re...
Text Solution
|
- For a reaction at equilibrium which of the following is correct ?
Text Solution
|
- Consider the reaction :- 2CO(g)+2H(2)O((g))hArr2CO(2(g))+2H(2(g))eq....
Text Solution
|
- For the following reaction in gaseous phase CO(g)+1/2O(2) rarr CO(2) K...
Text Solution
|
- Three moles of PCl(5), three moles of PCl(3) and two moles of Cl(2) ar...
Text Solution
|
- One mole of H(2) and 2 moles of I(2) are taken initially in a two lit...
Text Solution
|
- On doubling P and V at constant temperature, the equilibrium constant ...
Text Solution
|
- For the reaction, 2HI(g) rarr H(2)(g) + I(2) (g) - Q KJ , the equilibr...
Text Solution
|
- 1.6 mol of PCl(5)(g) is placed in 4 dm^(-3) closed vessel. When the te...
Text Solution
|
- Ammonium carbamate decomposes as : NH(2)COONH(4) (s) rarr 2NH(3)(g) ...
Text Solution
|
- 4 moles each of SO(2) "and" O(2) gases are allowed to react to form SO...
Text Solution
|
- In chemical equilibrium, the value of Delta n (number of molecules), i...
Text Solution
|