Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (7 MARK)|5 VideosETHERS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (A) Multiple Choice Type Questions |12 VideosETHERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (2 MARK)|19 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( Long Answer Type Questions ) |11 VideosEXAMINATION PAPER 2018
ARIHANT PUBLICATION|Exercise GROUP C|7 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ETHERS -QUESTIONS FOR PRACTICE (3 MARK)
- Compound A having molecular formula, C(4)H(10)O is found to be soluble...
Text Solution
|
- Illustrate with examples the limitations of the Williamson's synthesis...
Text Solution
|
- How is 1-propoxy propane synthesised from propan-1-ol ? Write the mech...
Text Solution
|
- Write the equation for the reaction of hydrogen iodide with 1-propox...
Text Solution
|
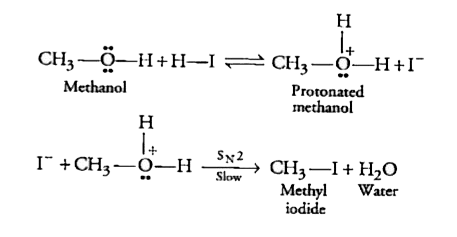
- Write the equation for the reaction of hydrogen iodide with methoxy ...
Text Solution
|
- Write the equation for the reaction of hydrogen iodide with benzyl e...
Text Solution
|
- The following is not an appropeiate reaction for the preparation of te...
Text Solution
|
- The following is not an appropeiate reaction for the preparation of te...
Text Solution
|
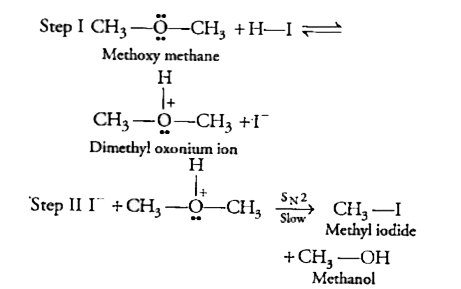
- Write the mechanism of the reaction of HI with methoxy methane.
Text Solution
|
- Explain the fact that in aryI alkyl ether, the alkoxy group activate...
Text Solution
|
- Explain the fact that in aryI alkyl ether, it directs the incoming s...
Text Solution
|