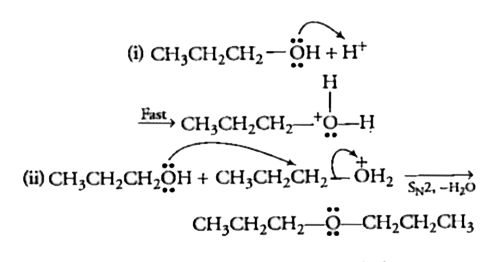
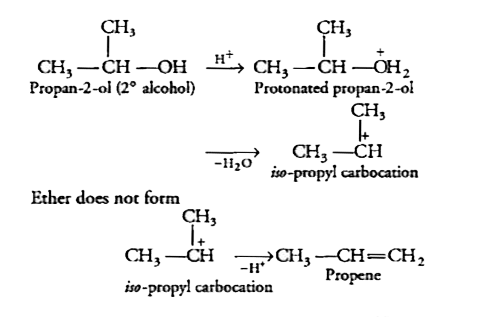
Text Solution
Verified by Experts
Topper's Solved these Questions
ETHERS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (A) Multiple Choice Type Questions |12 VideosETHERS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (B) Fill in the Blanks |8 VideosETHERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (3 MARK)|11 VideosELEMENTS : NITROGEN FAMILY
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( Long Answer Type Questions ) |11 VideosEXAMINATION PAPER 2018
ARIHANT PUBLICATION|Exercise GROUP C|7 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ETHERS -QUESTIONS FOR PRACTICE (7 MARK)
- Preparation of ethers by acid - catalysed dehydration of secondary and...
Text Solution
|
- Write equation of the following reactions. Friedel - Crafts reactio...
Text Solution
|
- Write equation of the following reactions. Nitration of anisole
Text Solution
|
- Write equation of the following reactions. Bromination of anisole in...
Text Solution
|
- Write equation of the following reactions. Friedel - Crafts (acetyla...
Text Solution
|