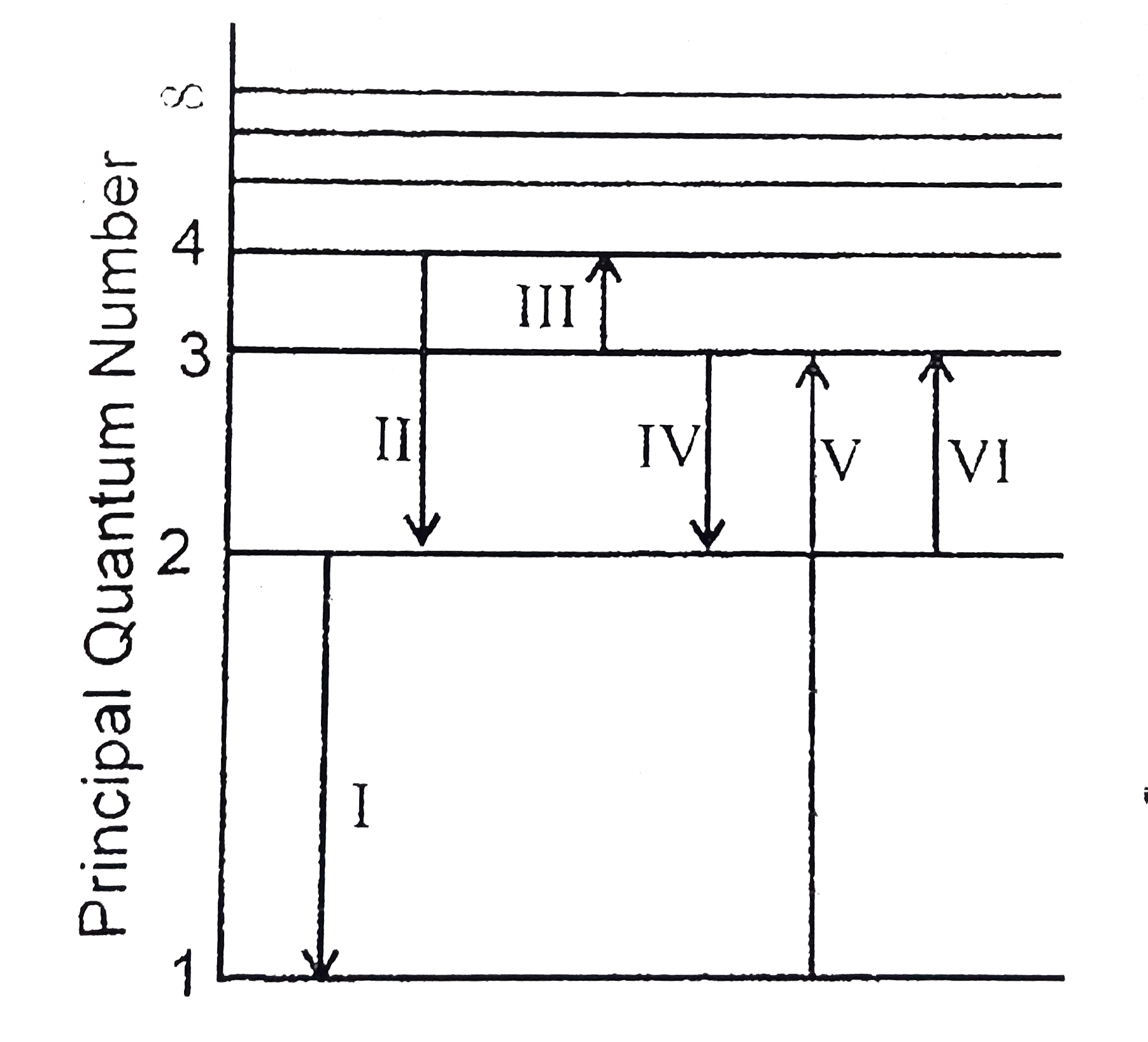
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise - 27|2 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise - 28|2 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise - 25|2 VideosRACE
ALLEN |Exercise Basic Maths (Wave Motion & Dopplers Effect) (Stationary waves & doppler effect, beats)|25 VideosTEST PAPER
ALLEN |Exercise PHYSICS|4 Videos
Similar Questions
Explore conceptually related problems