Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise Numerical Problems|69 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise ELECTROLYTIC CELLS AND ELECTROLYSIS|10 VideosCO-ORDINATION COMPOUNDS
BETTER CHOICE PUBLICATION|Exercise QUESTION FROM PREVIOUS BOARD EXAMINATION|59 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
BETTER CHOICE PUBLICATION|Exercise Question Bank (6.6 OXIDATION-REDUCTION)|15 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ELECTROCHEMISTRY-FUEL CELLS
- What are fuel cells ? Discuss H(2)-O(2) fuel cell. List some advantage...
Text Solution
|
- Which cell was used in Apollo space programme ? What was the product u...
Text Solution
|
- What is corrosion?
Text Solution
|
- Discuss the electrochemical theory of corrosion.
Text Solution
|
- CO(2) is always present in natural water. Explain its effect as rustin...
Text Solution
|
- Rusting of iron quicker in saline water than in ordinary water. Explai...
Text Solution
|
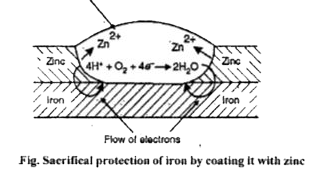
- How can iron be protected from rusting by sacrificial protection ?
Text Solution
|
- (a) What is galvanisation ? (b) Give three methods to protect iron f...
Text Solution
|