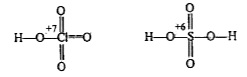
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Name the different oxoacids of halogens and draw their structure.
Text Solution
|
- Explain why fluorine forms only one oxoacid, HOF.
Text Solution
|
- Explain why perchloric acid is a strong acid than sulphuric acid.
Text Solution
|
- Arrange HCIO, HBrO and HIO in order to decreasing acidic strength givi...
Text Solution
|
- Compare the acidic strength of HClO4, HClO3, HClO2, HClO. Give reasons...
Text Solution
|
- Arrange HCIO4 , HCIO3, HCIO2, HCIO in order of oxidizing power. Give r...
Text Solution
|
- What are interhalogen compounds ? Give example.
Text Solution
|
- What are the interhalogen compounds ? Why are these more reactive than...
Text Solution
|
- Why ICI is more reactive than I2 ?
Text Solution
|
- Why ClF3 exists, but FCl3 does not exist ?
Text Solution
|
- Give important properties of magnets.
Text Solution
|
- Discuss the molecular shape of BrF3 on the basis of VSEPR theory.
Text Solution
|
- Draw the structure of following interhalogen compound. ClF3
Text Solution
|
- Draw the structure of following interhalogen compound. IF5
Text Solution
|
- Draw the structure of following interhalogen compound. IF7
Text Solution
|
- With what neutral molecule is CIO^- isoelectronic? Is that molecule a ...
Text Solution
|
- What are pseudohalogens ? Give example.
Text Solution
|
- What are freons ?
Text Solution
|
- Why are the elements of Group 18 known as noble gases ?
Text Solution
|
- Why elements of Group 18 are less reactive or inert?
Text Solution
|