Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-CO-ORDINATION COMPOUNDS-QUESTION FROM PREVIOUS BOARD EXAMINATION
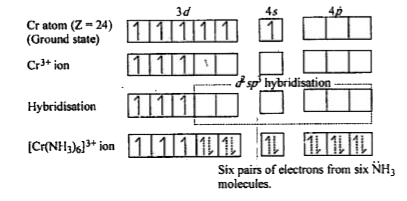
- Using valence bond theory of complexes, explain the geometry and diama...
Text Solution
|
- Write the IUPAC name of corordination compound [Co(NH3)3ONO]Cl2.
Text Solution
|
- How many isomers are possible for the netural complex [Co(NH3)3Cl3]? D...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Write the IUPAC name for the corordination compound. [Zn(NH3)4]2+
Text Solution
|
- Explain: [Ni (CN)4]^(2-) is diamagnetic while [Ni(Cl)4]^(2-) is parama...
Text Solution
|
- Write IUPAC name of the following K2[Zn(OH)4]
Text Solution
|
- Write IUPAC name of the following K3[Al(C2O4)]3
Text Solution
|
- Write IUPAC name of the following K2[Cu(CN4)]
Text Solution
|
- Write IUPAC name of the following [Co(NH3)3Cl]Cl2
Text Solution
|
- Write IUPAC name of the following [Co(en)2Br2Cl
Text Solution
|
- Write IUPAC name of the following [Co(NH3)5Br]Cl2
Text Solution
|
- Write IUPAC name of the following K3[Fe(C2O4)3]
Text Solution
|
- Write IUPAC name of the following Na[Au(CN)2]
Text Solution
|
- Write IUPAC name of the following K2[Fe(CN)6]
Text Solution
|
- Write IUPAC name of the following K2[Fe(CN)6]
Text Solution
|
- Write the IUPAC name of the following : K3[Fe (CN)5 NO]
Text Solution
|
- Write IUPAC name of the following K3[Cr(C2O4)3]
Text Solution
|
- Write IUPAC name of the following K3[Cr(C2O4)3]
Text Solution
|
- Write IUPAC name of the following K[PtCl3(NH3)]
Text Solution
|
- Write IUPAC name of the following K2[Fe(CN)6]
Text Solution
|