Text Solution
Verified by Experts
Topper's Solved these Questions
SOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION -D |16 VideosSOLVED SAMPLE PAPER, MARCH - 2021
ACCURATE PUBLICATION|Exercise SECTION - B |14 VideosSOLUTIONS
ACCURATE PUBLICATION|Exercise NUMERICAL QUESTIONS (3 MARKS)|31 VideosSOLVED MODEL TEST PAPER-1
ACCURATE PUBLICATION|Exercise SECTION D|14 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-SOLVED SAMPLE PAPER, MARCH - 2021 -SECTION - C
- Calculate the potential of hydrogen electrode income with a solution w...
Text Solution
|
- Compare and explain the reactivity of different alcohols towards sodiu...
Text Solution
|
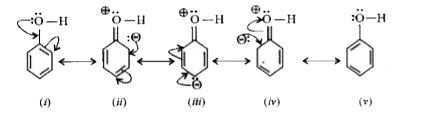
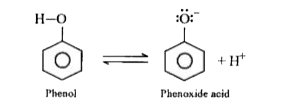
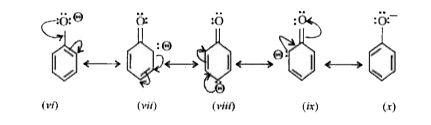
- Compare and explain the acidic nature of phenols .
Text Solution
|
- Show that the time required for 99% completion of a first order reacti...
Text Solution
|
- A first order reaction takes 40 min for 30% completion. Calculate t(1/...
Text Solution
|
- Why is dioxygen gas but sulphur a solid?
Text Solution
|