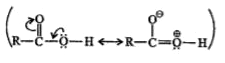A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID & THEIR DERIVATIVES
BRILLIANT PUBLICATION|Exercise LEVEL-II|47 VideosCARBOXYLIC ACID & THEIR DERIVATIVES
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|10 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION-REASON TYPE QUESTIONS)|6 VideosCARBOXYLIC ACIDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion -Reason)|4 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CARBOXYLIC ACID & THEIR DERIVATIVES-LEVEL-III (Linked Comprehension Type)
- Acids do not give the characteristic reaction of (C=0) group because o...
Text Solution
|
- Paragraph An organic compound A(C(9)H(6)O(3)) does not react with...
Text Solution
|
- Paragraph An organic compound A(C(9)H(6)O(3)) does not react with...
Text Solution
|
- Paragraph An organic compound A(C(9)H(6)O(3)) does not react with...
Text Solution
|
- Paragraph Dicarboxylic acids have two carboxylic groups, eg., ...
Text Solution
|
- Paragraph Dicarboxylic acids have two carboxylic groups, eg., ...
Text Solution
|
- Paragraph Dicarboxylic acids have two carboxylic groups, eg., ...
Text Solution
|
- . Which of the following has the smallest pK(a) value?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- (I) C(6)H(5) COOH (II) p-C(6)H(4)(OH) (COOH) (III) p-(NO(2)) C(6)...
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- Paragraph The acidic strength of saturated aliphatic carboxylic aci...
Text Solution
|
- Paragraph The acidic strength of saturated aliphatic carboxylic aci...
Text Solution
|