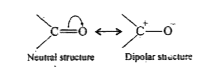Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.5 Uses Of Aldehydes And Ketones)|3 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.6 Nomenclature And Structure of Carboxylic Group)|8 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.2 Preparation of Aldehydes And Ketones )|10 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12. 3 Physical Properties )
- Why do aldehydes and ketones have high dipole moments?
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- Aldehydes have lower boiling points than the corresponding alcohols. E...
Text Solution
|
- Sodium bisulphite is used for the purification of aldehydes and ketone...
Text Solution
|
- Write Clemmensen reduction.
Text Solution
|
- Carbonyl compounds mainly show nucleophilic addition. reactions. Why?
Text Solution
|
- Aldehydes are more reactive than Ketones. Explain.
Text Solution
|
- Formaldehyde is more reactive than other aldehydes.Justify.
Text Solution
|
- Give aldol condensation reaction of acetaldehyde and explain why forma...
Text Solution
|
- Write Aldol condensation reaction.
Text Solution
|
- Write cross aldol condensation.
Text Solution
|
- Write cross aldol condensation.
Text Solution
|
- Write Claisen condensation.
Text Solution
|
- Aldehydes can be used to make ethyl acetate. Write chemical equation. ...
Text Solution
|
- How acetaldehyde can be used to ethyl acetate ?
Text Solution
|
- Benzaldehyde does not undergo Aldol condensation. Give reason only.
Text Solution
|
- What are Kolbe’s Reaction ? Give one example.
Text Solution
|
- How will you distinguish between : CH(3)CHO (acetaldehyde or propana...
Text Solution
|
- How will youdistinguish between benzaldehyde andacetaldehyde?
Text Solution
|