Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.8 Chemical Reaction)|49 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.9 Uses of Carboxylic Acids)|4 VideosORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II
BETTER CHOICE PUBLICATION|Exercise Question Bank (12.6 Nomenclature And Structure of Carboxylic Group)|8 VideosHALOALKANES AND HALOARENES
BETTER CHOICE PUBLICATION|Exercise QUESTIONS|158 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ORGANIC COMPOUND WITH FUNCTIONAL GROUP CONTANING OXYGEN-II-Question Bank (12.7 Physical properties)
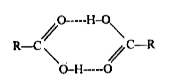
- Carboxylic acid exists as dimers, explain why ?
Text Solution
|
- Most aromatic acids are solids while acetic acid and others of their s...
Text Solution
|
- Most aromatic acids are solids while acetic acid and others of their s...
Text Solution
|
- Why are the boiling points of carboxylic acids higher than the corresp...
Text Solution
|