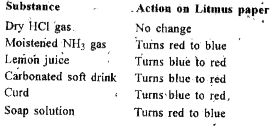Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES AND SALTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Long Answer Type Questions)|6 VideosACIDS, BASES AND SALTS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS (Very Short Answer Type Questions)|8 VideosCARBON AND ITS COMPOUNDS
SWAN PUBLICATION|Exercise ADDITIONAL IMPORTANT QUESTIONS(LONG ANSWER TYPE QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-ACIDS, BASES AND SALTS-ADDITIONAL IMPORTANT QUESTIONS ( Short Answer Type Questions)
- What will be the action of the following substances on litmus paper ? ...
Text Solution
|
- Name the acid present in ant sting and give its chemical formula. Also...
Text Solution
|
- What happens when nitric acid is added to egg shell?
Text Solution
|
- What is the importance of transpiration?
Text Solution
|
- is the liquid part of the blood which is yellowish in colour.
Text Solution
|
- Which gas is usualy libertad when an acid reacts with a metal? Illustr...
Text Solution
|
- are disc shaped cells containing a red coloured pigment called haemogl...
Text Solution
|
- is the component of blood which is known as fighting cell.
Text Solution
|
- Explain how the pH change is the cause of tooth decay?
Text Solution
|
- What is the importance of blood in the body?
Text Solution
|