Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SWAN PUBLICATION-ACIDS, BASES AND SALTS-ADDITIONAL IMPORTANT QUESTIONS (Long Answer Type Questions)
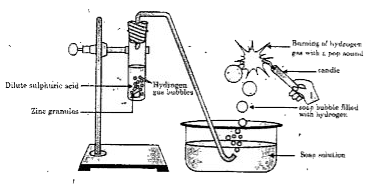
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- In the following schematic diagram for the preparation of hydrogen gas...
Text Solution
|
- A dry pellet of a common base B, when kept in open absorbs moisture an...
Text Solution
|
- A red pigment called is responsible for the red colour of the blood.
Text Solution
|