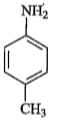
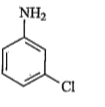
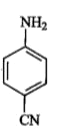
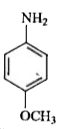
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Numerical Type)|5 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Matching Column Type)|5 VideosNITROGEN COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-III (Single Correct Answer Type)|7 VideosHALOALKANES AND HALOARENES
BRILLIANT PUBLICATION|Exercise Level-II|37 VideosNUCLEAR CHEMISTRY
BRILLIANT PUBLICATION|Exercise LEVEL-III (Linked Comprehension Type) |11 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-NITROGEN COMPOUNDS-LEVEL-III (Multiple Choice Answer Type)
- Which of the following reaction(s) will give nitrobenzene?
Text Solution
|
- Diazonium salt of which of the following substituted aromatic amine un...
Text Solution
|
- Which of the following reagent(s) can be used for protection of amine(...
Text Solution
|
- When the dehydration of acetamide to acetonitrile occurs, which of the...
Text Solution
|
- By reacting with which of the following reagents CH3 NH2 will form a s...
Text Solution
|
- Which of the following statements is/are true
Text Solution
|
- Which of the following give primary amine on reduction?
Text Solution
|
- The reagents used to distinguish between (CH3)3 N ,(CH3)2 NH is/are
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- A negative carbylamine test is given by
Text Solution
|
- Examine the following structures for the anilinium ion, and choose the...
Text Solution
|