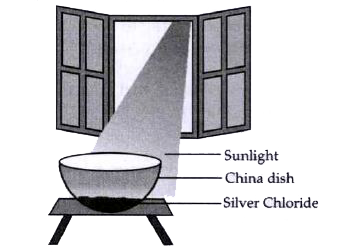
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT-2 (Assertion and Reason Type Questions) |2 VideosCHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT-2 (Very Short answer Type Questions) |3 VideosCHEMICAL REACTIONS
OSWAL PUBLICATION|Exercise SELF ASSESSMENT-2 (Multiple Choice Questions) |3 VideosCARBON COMPOUNDS
OSWAL PUBLICATION|Exercise BOARD CORNER ( Long Answer Type Questions)|4 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAL PUBLICATION|Exercise CASE-BASED MCQs |29 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-CHEMICAL REACTIONS-SELF ASSESSMENT-2 (Passage / Diagram Based Questions)
- The following diagram displays a chemical reaction. Observe carefully ...
Text Solution
|
- The following diagram displays a chemical reaction. Observe carefully ...
Text Solution
|
- The following diagram displays a chemical reaction. Observe carefully ...
Text Solution
|
- The following diagram displays a chemical reaction. Observe carefully ...
Text Solution
|