A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
EDUCART PUBLICATION-SAMPLE PAPER 5-SECTION C
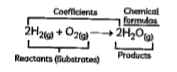
- Case 1: A chemical equation is the representation of chemical change i...
Text Solution
|
- Case 1: A chemical equation is the representation of chemical change i...
Text Solution
|
- Case 1: A chemical equation is the representation of chemical change i...
Text Solution
|
- Case 1: A chemical equation is the representation of chemical change i...
Text Solution
|