To describe an electron completely four quantum numbers were predicted. They are
1. Principal quantum number n
2.Azimuthal quantum number, l
3. Magnetic quantum numbe, `m_(i)` and
4. Spin quantum number `m_(s)`.
a. Principal Quantum Number:
1. This was proposed by Neils Bohr.
1. This was proposed by Neils Bohr.
2. It is denoted by the letter n.
3. It represents the circular orbits around the nucleus.
4 As the vlaue of n increases the size and energy of the orbit increases.
5. According to number method n has the values 1,2,3........................According to letter method n can be represented by the letters K,L,M...........
6. In any orbit the number of sub orbits=n, number of orbitals = `n^(2)`
number of electrons `=2n^(2)`
7. This Quantum number describes the size and energy of the orbit.
b. Azimuthal Quatum Number
1. This was proposed by Sommerfeld.
2. It is also known as Angular momentum quantum number.
It is denoted by the letter l.
4.This quantum number represents the sub levels present in the main levels.
5 The sub levels are s,p,d and f
6. The l values of s,p, d and f sub levels are 0,1,2 and 3 respectievely.
7. The first main level contains only one sub level and it is s. The second main level contains s,p sub levesl. The third main level contains s,p,d sub levels. The fourth main level contains s,p,d and f sub levels.
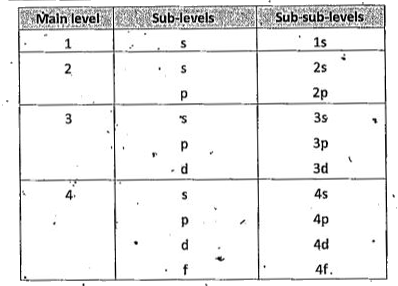
8. The relative energy values of 1s, 2s, 2p etc. can be calculate by adding up their n and l values.
Ex: 1.Energy values of `1s=1+0=1`
2. Energy value of `2p=2+1=3`
3.Energy values of `3s=3+2=5`
4. Energy value of `4f=4+3=7`
9. This Quantum number of describes the shape of the orbital.

c. Magnetic Quantum Number:
1. This was proposed by Lande.
2. It is denoted by the letter m.
3. This quantum number describes the sub sub level or orbitals present in a givensub level.
4.`m_(l)` has values from -l to + l through O.
5. The total number of m values for a given of l is `(2l+1)`.

6. All theorbitals present in a given sub level possess the same energy values, because they possess the same n and l values.
7. This quantum number describes the orientation of the orbitals in space. 1. It was proposed by Uhlenbeck and Guodsmit.
2. It is denoted by `m_(s)`.
3. This quantum number describes the spirit of the revolving electron.
4. `m_(s)` value of clockwise electron is `+1//2` and that of anticlockwise electron is `-1//2`.
5. Clockwise revolving electron is repre sented by `+1//2` and anticlockwise revo lying electron is represented by `-1//2`.
6. This quantum number describes the direction of spin of the revolving electron.


