A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-PRACTICE SET 03-PAPER 1 (PYSICS & CHEMISTRY)
- The Temperature at which 28 g of N(2) will occupy a volume of 10.0 L a...
Text Solution
|
- A solution of two liquids boils at a temperature more than the boiling...
Text Solution
|
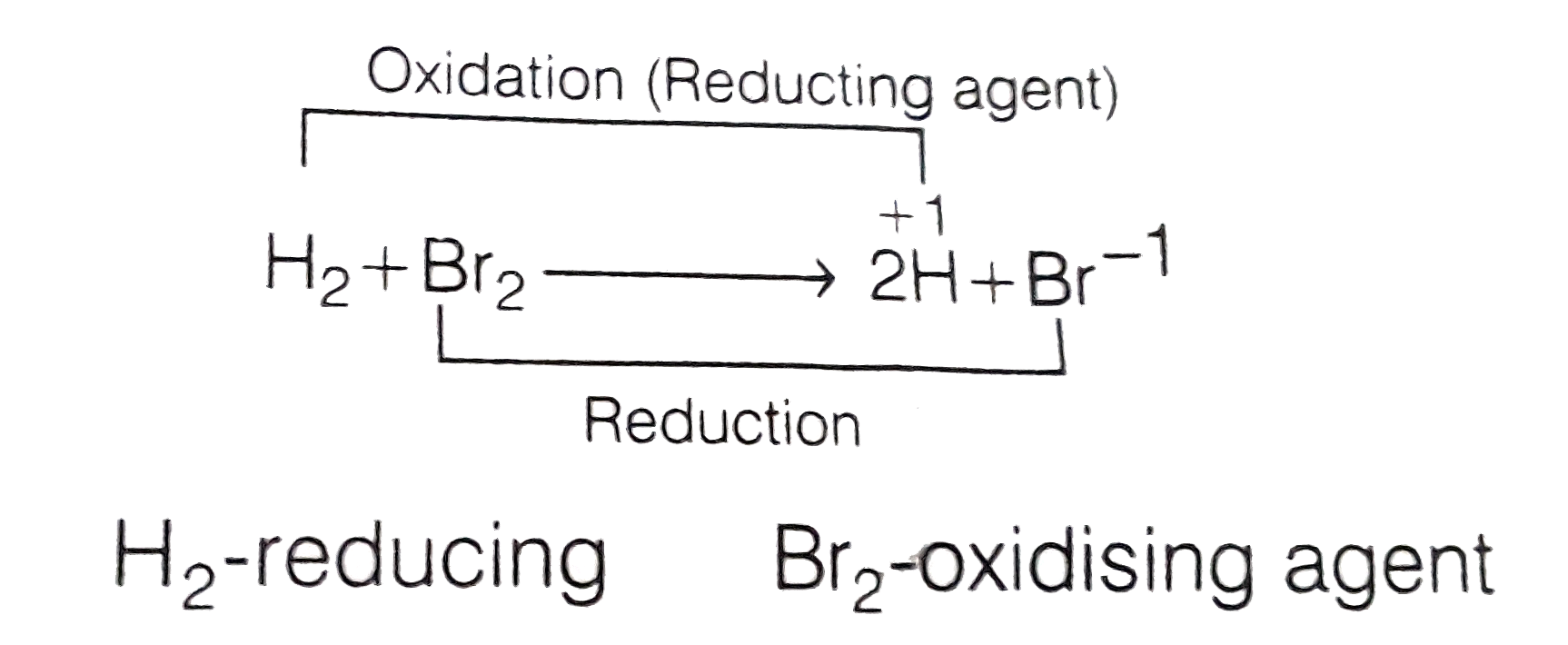
- Both oxidation and reduction takes place in
Text Solution
|
- By diluting a weak electrolyte, specific conductivity (K(c)) and equiv...
Text Solution
|
- A first orde reaction is 75% completed after 32min. When was 50% of th...
Text Solution
|
- A sample of H(2)O(2) is labelled 10 vol. Its percentage strength will ...
Text Solution
|
- The solubility of Na(2),SO(4),BeSO(4),MgSO(4) and BaSO(4) will follow ...
Text Solution
|
- The statement, which prompted Neil Bartlett to prepare the first noble...
Text Solution
|
- Radioactivity is the phenomenon most common with the
Text Solution
|
- Gammexane is
Text Solution
|
- When acetylene is passed through di. H(2)SO(4) in presence of HgSO(4),...
Text Solution
|
- Which isomer of hexane has only two different sets of structuraly equi...
Text Solution
|
- How many mL of perhydrol is requried to produce sufficient oxygen whic...
Text Solution
|
- Which of the following statements is incorrect about coordination comp...
Text Solution
|
- When phenol is distilled with zinc dust, it gives
Text Solution
|
- To correct the order of basic nature is
Text Solution
|
- Reaction of nitrous acid wiith aliphatic primary aminie in cold gives
Text Solution
|
- Identify A and B in the reaction given below Ethane nitrile underset...
Text Solution
|
- On heating with conc. HNO3, proteins give yellow colour. This test is ...
Text Solution
|
- Correct statement among the following is
Text Solution
|