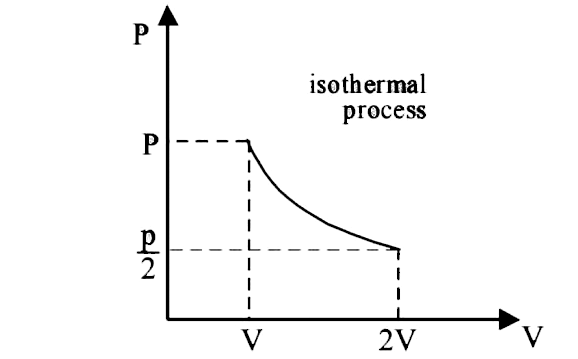
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- Three closed vessels A, B and C are at the same temperature T and cont...
Text Solution
|
- An ideal gas is taken from the state A (pressure P, volume V) to the s...
Text Solution
|
- Two bodies A and B ahave thermal emissivities of 0.01 and 0.81 respect...
Text Solution
|
- The temperature of an ideal gas is increased from 120K to 480K. If at ...
Text Solution
|
- A given quantity of a ideal gas is at pressure P and absolute tempera...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure,
Text Solution
|
- A blackbody is at a temperature of 2880K. The energy of radiation emi...
Text Solution
|
- A bimetallic strip is formed out of two identical strips one of copper...
Text Solution
|
- Two identical containers A and B with frictionless pistons contain the...
Text Solution
|
- Let barv,v(rms) and vp respectively denote the mean speed. Root mean s...
Text Solution
|
- A vessel contains a mixture of one mole of oxygen and two moles of nit...
Text Solution
|
- A black body of temperature T is inside chamber of T0 temperature init...
Text Solution
|
- Cv and Cp denote the molar specific heat capacities of a gas at costan...
Text Solution
|
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cylic process ...
Text Solution
|
- A composite block is made of slabs A,B,C,D and E of different thermal ...
Text Solution
|
- The figure below shows the variation of specific heat capacity (C) of ...
Text Solution
|
- A container of fixed volume has a mixture of a one mole of hydrogen an...
Text Solution
|