A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2005-CHEMISTRY
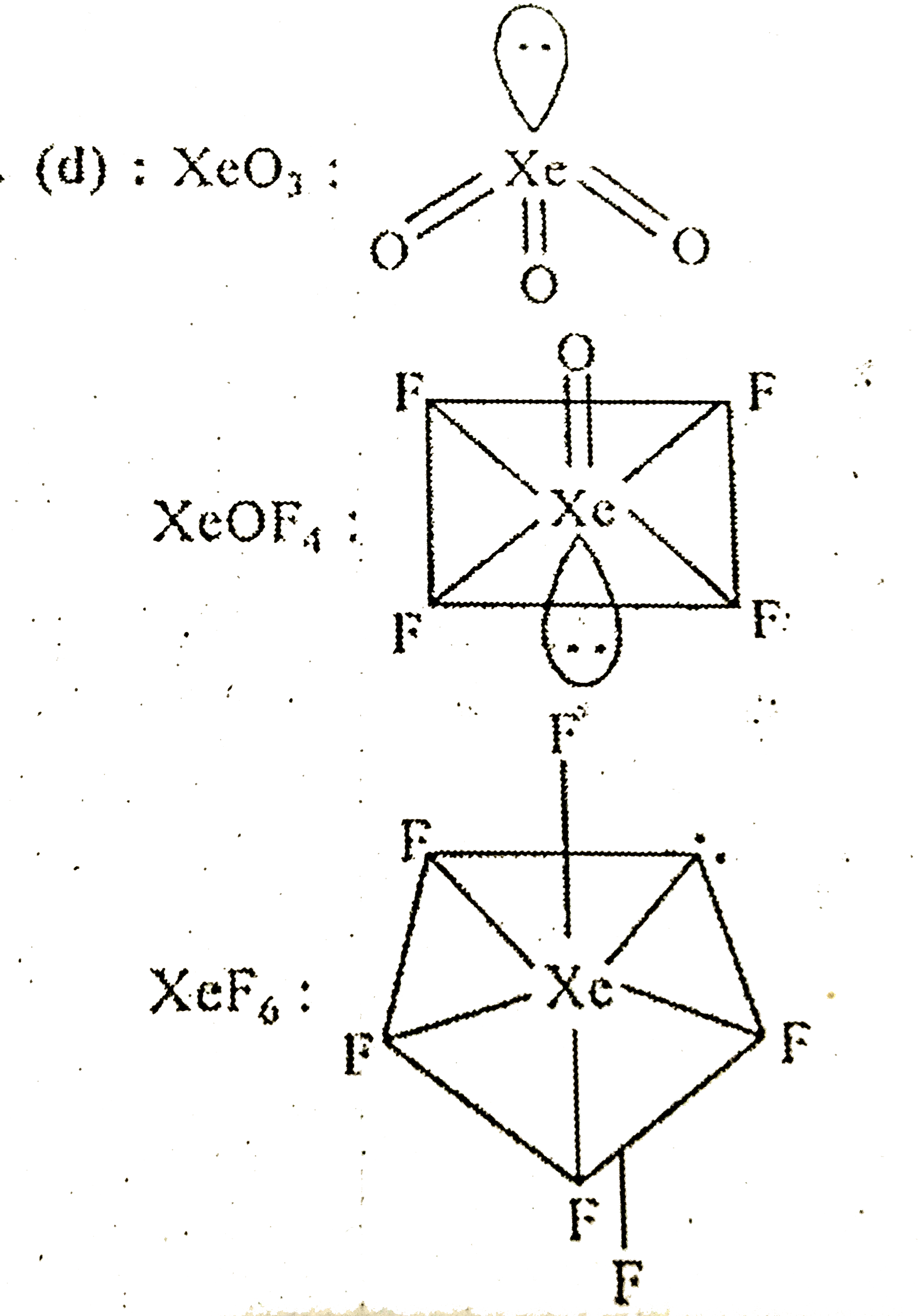
- Among the following molecules, (i)XeO(3)(ii)XeOF(4)(iii)XeF(6) those h...
Text Solution
|
- An aqueous solution of COCL2 on addition of excess of concentrated HCl...
Text Solution
|
- In which of the following pairs both the complex show optical isomeris...
Text Solution
|
- The diamagnetic species is
Text Solution
|
- In the balanced chemical reaction IO(3)^(ө)+al^(ө)+bH^(ө)rarrcH(2)O+...
Text Solution
|
- Among the following pairs of ions the lower oxidation state in aqueous...
Text Solution
|
- The number of P-O-P bridge in the structure of phosphorous pentoxide a...
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- Which of the following gives propyne on hydrolysis ?
Text Solution
|
- The pair of amphoteric hydroxides is
Text Solution
|
- Which of the following is a carbonate ore?
Text Solution
|
- .(92)U^(238) emits 8 alpha- particles and 6 beta- particles. The n//p ...
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|
- F(2) is formed by reacting K(2) MnF(6) with
Text Solution
|
- The isoeletronic pair is
Text Solution
|
- Which of the following chemicals are used to manufacture methy1 isocya...
Text Solution
|
- alpha-Particles can be detected using
Text Solution
|
- Which of the following molecules is most suitable to disperse benzen i...
Text Solution
|
- The chemical reaction 2AgCl("(fused)")+H(2(g))rarr2HCl((aq))+2Ag((s)...
Text Solution
|
- If 'Z' is the number of atoms in the unit cell that represents the cl...
Text Solution
|