A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2004-Chemistry
- Which of the following is only acidic in nature?
Text Solution
|
- Which one of the following forms with an excess of CN^(-) (Cyanide) a ...
Text Solution
|
- Which of the following is not considered as an organometallic compound...
Text Solution
|
- Dimethyl glyoxime gives a red precipitate with Ni^(2+), which is used ...
Text Solution
|
- The element which forms oxides in all oxidation states +1 to +5 is.
Text Solution
|
- For decolourisation of 1 "mol of" KMnO(4), the moles of H(2)O(2) requi...
Text Solution
|
- The statement true for N(3)^(-) is
Text Solution
|
- Which of the following does not have optical isomer?
Text Solution
|
- For the electron affinity of halogens (with -ve sign), which of the fo...
Text Solution
|
- Shape of O(2)F(2) is similar to that of
Text Solution
|
- The liquified metal expanding on solidification is :
Text Solution
|
- The compound insoluble in water is
Text Solution
|
- Which of the following imparts colour to the burner flame?
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- Among the following the dissociation constant is highest for
Text Solution
|
- The strongest base among the following .
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Among the following, the aromatic compound is
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- The geometrical isomerism is shown by:
Text Solution
|
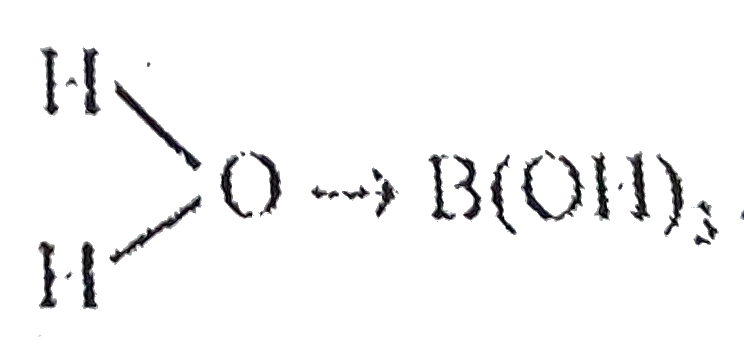 .In this species,`B^(3+)` ion,because of its small size,exercises a high polarizing power thereby pulling the sigma electeron charge of the coordinated O atom towards itself.The coordinated oxygen,in turn,pulls the sigma electron charge of the OH bond of the attached `H_(2)O` molecule towards itself.This facilitates the removal of `H^(+)` ion from the O-H bonds,as shown below
.In this species,`B^(3+)` ion,because of its small size,exercises a high polarizing power thereby pulling the sigma electeron charge of the coordinated O atom towards itself.The coordinated oxygen,in turn,pulls the sigma electron charge of the OH bond of the attached `H_(2)O` molecule towards itself.This facilitates the removal of `H^(+)` ion from the O-H bonds,as shown below