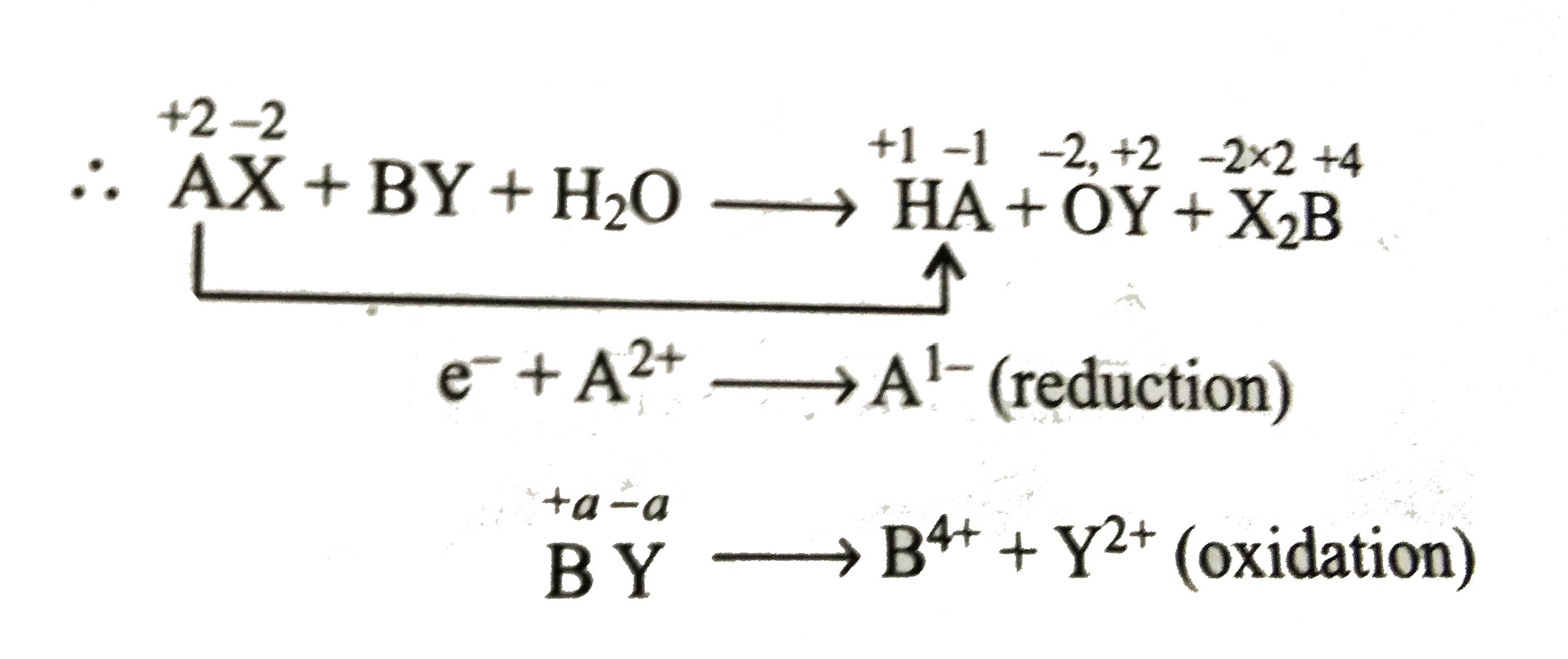A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|32 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|91 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercise|29 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDOX REACTIONS-Exercises (Linked Comprehension)
- Consider the following unbalanced redox reaction: H(2)O+AX+BYrarrHA+...
Text Solution
|
- Consider the following unbalanced redox reaction: H(2)O+AX+BYrarrHA+...
Text Solution
|
- Consider the following unbalanced redox reaction: H(2)O+AX+BYrarrHA+...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- Oxidation reaction involves loss of electrons, and reduction reaction ...
Text Solution
|
- The valancy of carbons is generally 4, but its oxidation state may be ...
Text Solution
|
- The valancy of carbons is generally 4, but its oxidation state may be ...
Text Solution
|
- The valancy of carbons is generally 4, but its oxidation state may be ...
Text Solution
|
- The valancy of carbons is generally 4, but its oxidation state may be ...
Text Solution
|
- The valancy of carbons is generally 4, but its oxidation state may be ...
Text Solution
|
- Redox equations are balanced either by ion-electron method or by oxida...
Text Solution
|
- Redox equations are balanced either by ion-electron method or by oxida...
Text Solution
|
- Redox equations are balanced either by ion-electron method or by oxida...
Text Solution
|
- Redox equations are balanced either by ion-electron method or by oxida...
Text Solution
|
- Cartain materials such as turpentine oil, unsaturated organic compound...
Text Solution
|
- Cartain materials such as turpentine oil, unsaturated organic compound...
Text Solution
|
- Cartain materials such as turpentine oil, unsaturated organic compound...
Text Solution
|