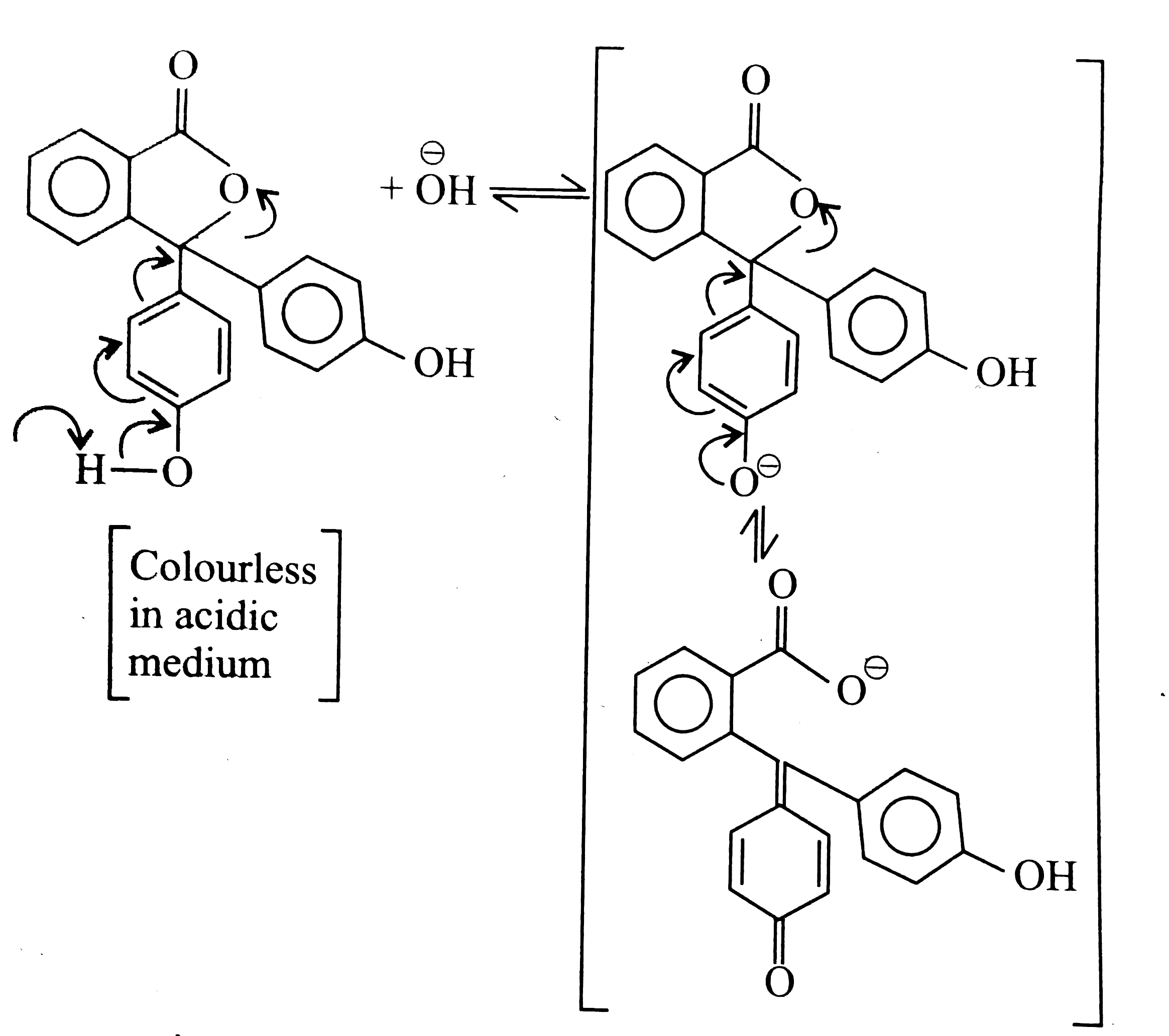
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|36 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Integer|10 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|33 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-IONIC EQUILIBRIUM-Exercises Single Correct
- A solution of 0.1M NaZ has pH = 8.90. The K(a) of HZ is
Text Solution
|
- Phenolphalein does not act as an indicator for the titration between
Text Solution
|
- The pink colour of phenolphthalein in alkaline medium is due to
Text Solution
|
- Methy1 orange gives red colour in
Text Solution
|
- A solution containing NH(4)CI and NH(4)OH has [overset(Theta)OH] = 10^...
Text Solution
|
- If equal volumes of BaCI(2) and NaF solutions are mixed, which of thes...
Text Solution
|
- The solubility of solid silver chromate, Ag(2)Cro(4), is determined in...
Text Solution
|
- The solubility products of AI(OH)(3) and Zn(OH)(2) are 8.5xx10^(-23) a...
Text Solution
|
- If K(sp) (PbSO(4)) =1.8 xx 10^(-8) and K(a) (HSO(4)^(Theta)) = 1.0 xx ...
Text Solution
|
- Which one of the following is true for any diprotic acid, H(2)X?
Text Solution
|
- The K(sp) of Mg(OH)(2) is 1xx10^(-12). 0.01M Mg^(2+) will precipitate ...
Text Solution
|
- The solubility products of MA, MB, MC and MD are 1.8xx10^(-10), 4xx10^...
Text Solution
|
- A solution is saturated with respect to SrCO(3) and SrF(2). The [CO(3)...
Text Solution
|
- The number of S^(2-) ions present in 1L of 0.1 MH(2)S [K(a(H(2)S)) = 1...
Text Solution
|
- The solubility of AgI in NaI solutions is less than that in pure water...
Text Solution
|
- Three sparigly soluble salts M(2)X, MX,and MX(3) have the same solubil...
Text Solution
|
- When 0.2M solution of acetic acid is neutralised with 0.2M NaOH in 500...
Text Solution
|
- A weak acid HX has the dissociation constant 1 xx 10^(-5)M. It forms a...
Text Solution
|
- A certain buffer solution contains equal concentartion of X^(Theta) an...
Text Solution
|
- A certain weak acid has a dissocation constant of 1.0 xx 10^(-4). The ...
Text Solution
|