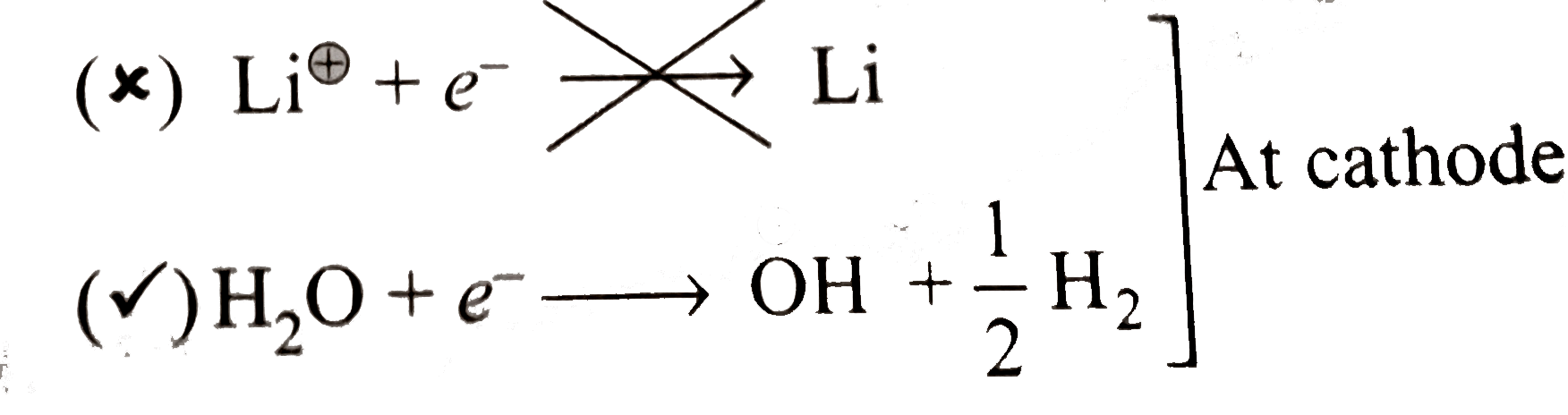Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisetrue / False|40 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves (Linked Comprehension )|13 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exerciseinterger|8 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exercisefill In The Blanks
- When an aqueous solution of LiCl is electrolyzed using graphite electr...
Text Solution
|
- Given E^(c-).(Fe^(2+)|Fe) and E^(c-).(Fe^(3+)|Fe^(2+)) are -0.44 and 0...
Text Solution
|
- A certain current liberates 0.504g of H(2)(g) in 2 hours. The weight o...
Text Solution
|
- Number of Faradays required to reduce 3 mol of MnO(4)^(c-) to MnO^(2+)...
Text Solution
|
- The gasX at 1 atm is bubbled through a solution containing a mixture o...
Text Solution
|
- The EMF of the cell : Pt, H(2)(1atm)|H^(o+)(aq)||AgCl|Ag is 0.27 and...
Text Solution
|
- The electrochemical equivalent for zinc ( atomic weight =6.4) is ……………...
Text Solution
|
- Given : Fe^(2+)(aq)+2e^(-) rarr Fe(s), " "E^(c-)=-0.44V Al^(3)+...
Text Solution
|
- When electricity is passed through a solution of AlCl(3) and 13.5g of ...
Text Solution
|
- A current of 2A is passed for 1.93 xx 10^(4)s through a molten tin sa...
Text Solution
|
- 13g of metal M is deposited at cathode by passing 0.4F of electricity ...
Text Solution
|
- If the temperature coefficient ((delE)/(delT)) is zero for a cell rea...
Text Solution
|
- The ionization constant (K(a)) of a weak electrolyte is 2.5xx10^(-7), ...
Text Solution
|
- When dilute H(2)SO(4) is electrolyzed between Pt electrodes, the gas l...
Text Solution
|
- The amount of substance liberated when 1 ampere of current is passed ...
Text Solution
|
- The amount of charge carried by N^(3-) ion is …………………………
Text Solution
|
- The current is carried through metallic conductor by ……………………….. and i...
Text Solution
|
- Electrolytic conductance ………………………. with increase of temperature while...
Text Solution
|
- According to the preferential discharge theory, out of a number of ion...
Text Solution
|
- At infinite dilution of an electrolyte, the equivalent conductance of ...
Text Solution
|