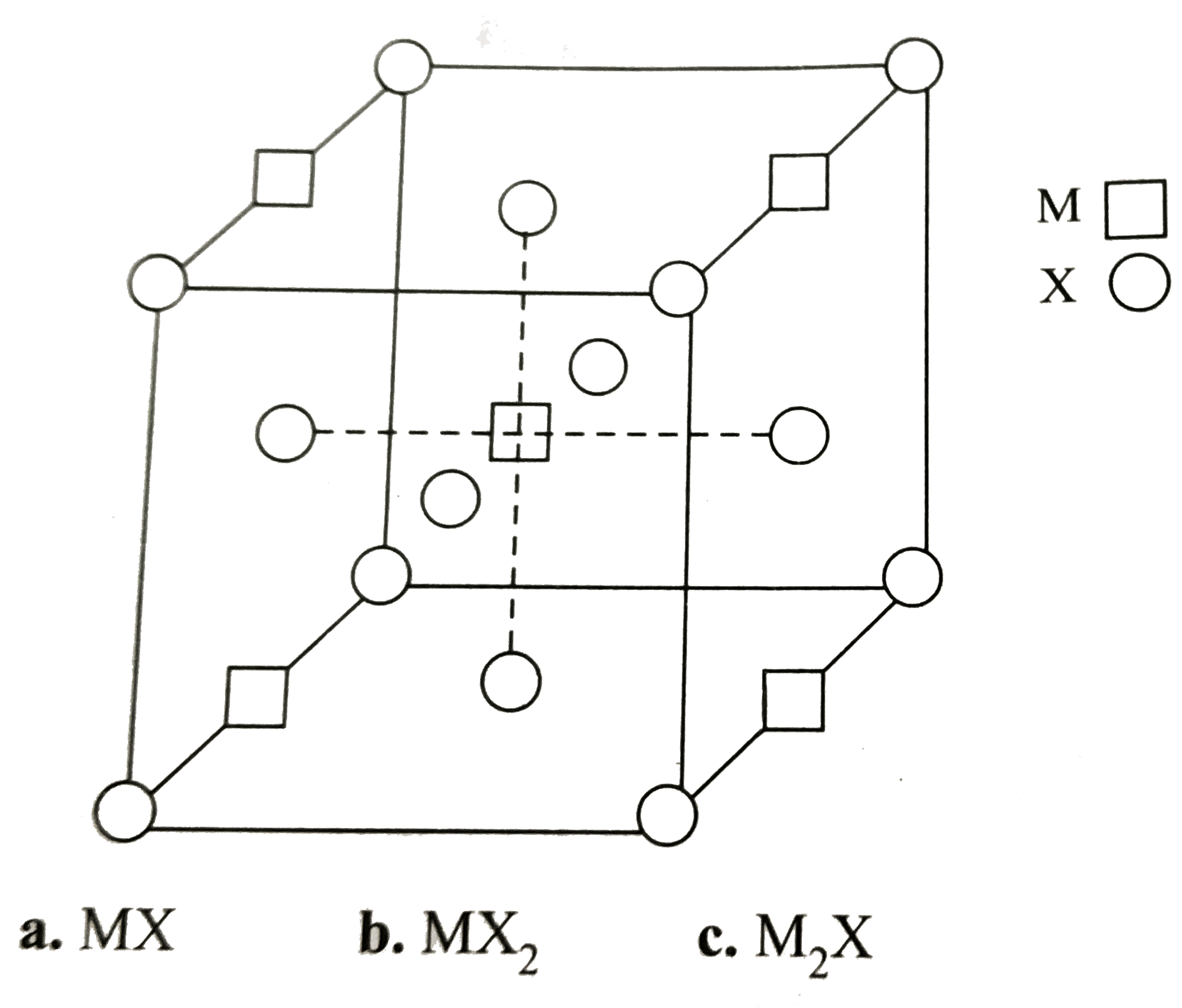
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THE SOLID STATE -Higher Order Thinking Skills
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- In a close packed structure of mixed oxides , the lattice is composed ...
Text Solution
|
- Match the type of packing given in column I with the iterms give...
Text Solution
|
- The density and edge length values for a crystalline element with fcc ...
Text Solution
|
- For two isomorphous crystals A and B , the ratio of density of A to th...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- The density of mercury is 13.6 g mL^(-1). Calculate the approximate di...
Text Solution
|
- An element crystallizes into a structure which may be describes by a c...
Text Solution
|
- A sample of ferrous oxide has actual formula Fe(0.93)O(1..00). In this...
Text Solution
|