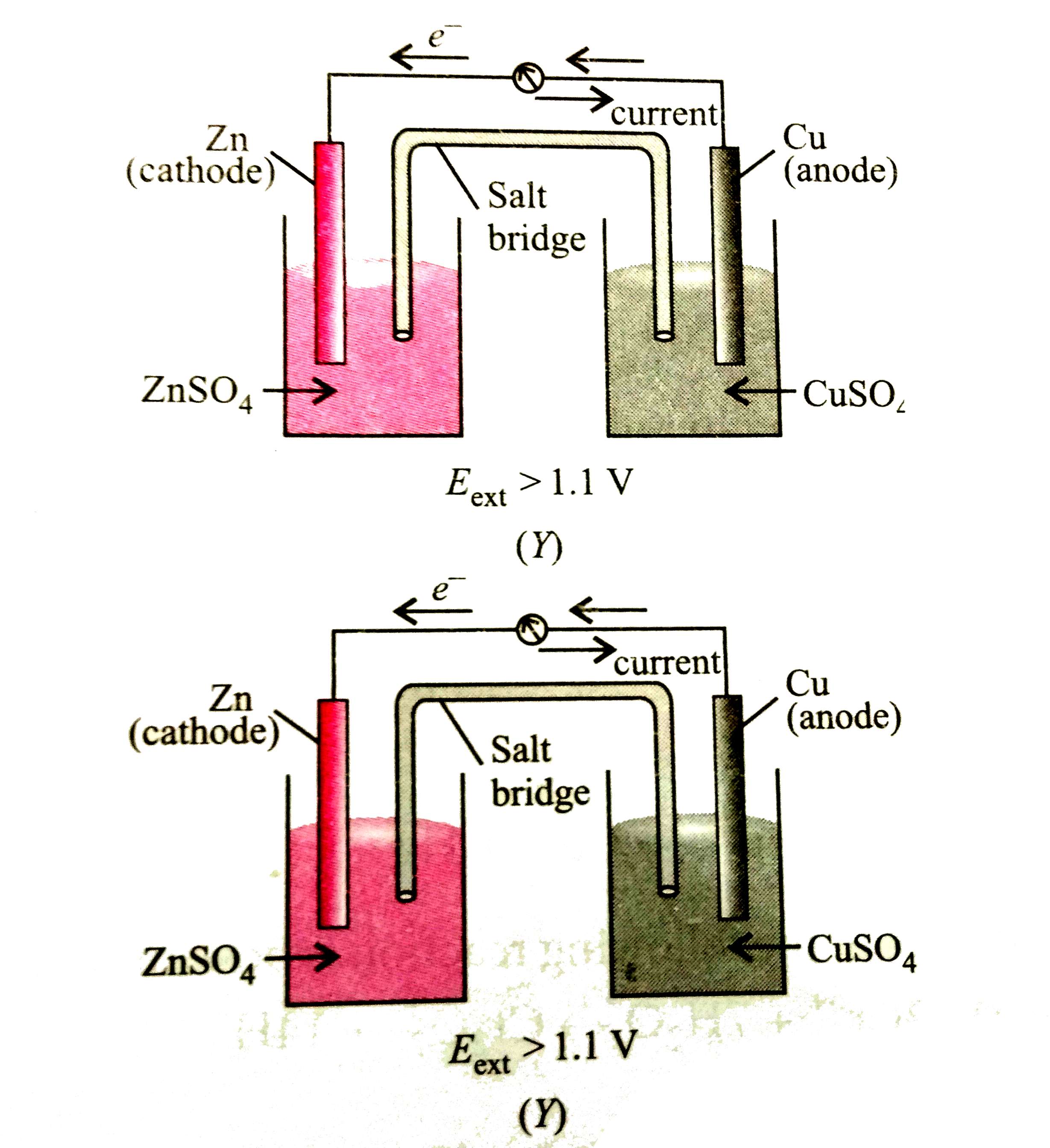A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Galvanic Cell|19 VideosELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Nernst Equation|17 VideosELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-ELECTROCHEMISTRY-MCQs
- Given below are two figures of Daniell cell (X) and (y). Study the fig...
Text Solution
|
- A cell is set up as shown in the figure. It is observed that EMF of th...
Text Solution
|
- Label the parts represented by (A), (B) and C
Text Solution
|
- Which of the given statements for mercury cell are incorrect? (i)...
Text Solution
|
- Label the given diagram showing lead storage battery :
Text Solution
|