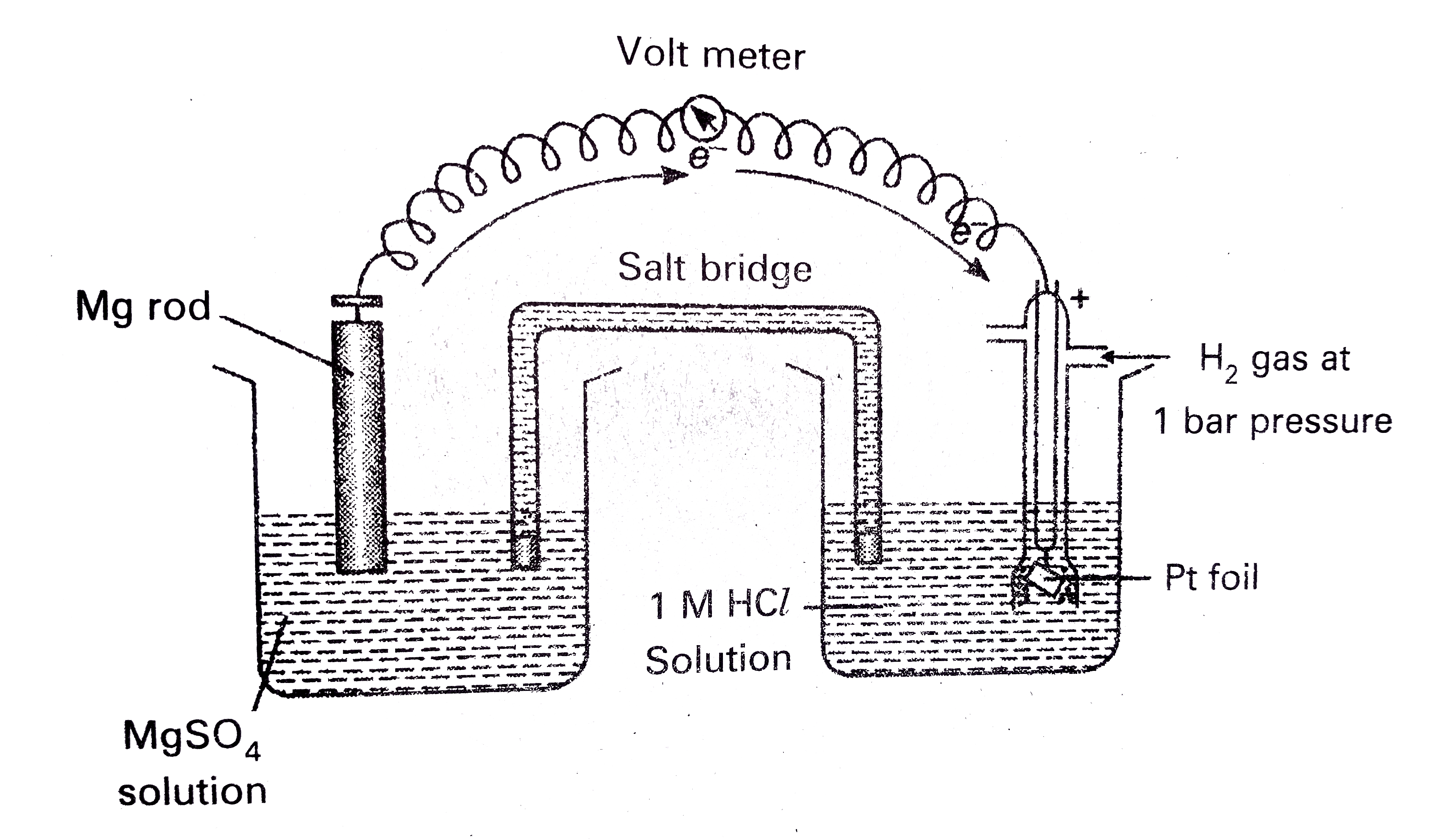
Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|12 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-INTEXT QUESTIONS
- How would you determine the standard electrode potential of the system...
Text Solution
|
- Can you store copper sulphate soolutions in a zinc pot ?
Text Solution
|
- Consult the table on standard electrode potentials and suggest three s...
Text Solution
|
- Calculate the potential of hydrogten electrode placed in a solution of...
Text Solution
|
- Calculate the emf of the cell with the cell reaction Ni ((s))+2Ag ^(...
Text Solution
|
- The cell in which the following cell reaction occurs, 2Fe ((aq))^(3+...
Text Solution
|
- Why does the conductivity of a solution decrease with dilution ?
Text Solution
|
- Suggest a way to determine the Lambdam^0 value of water .
Text Solution
|
- The molar conductivity of 0.025mol L^(-1) methanoic acid is 46.1 S cm^...
Text Solution
|
- If a current of 0.5 ampere flows through a metallic wire for 2h, then ...
Text Solution
|
- Suggest a list to metals that are extracted electrolytically.
Text Solution
|
- Consider the reaction, Cr(2)O(7)^(2-) +14H^(+) +6e^(-) to 2Ce ^(3+) ...
Text Solution
|
- Write the chemistry of recharging the lead stronge battery, highlighti...
Text Solution
|
- Suggest two materials other than hydrogen that can be used as fuels in...
Text Solution
|
- Explain how rusting of iron is envisaged as electrochemical cell.
Text Solution
|
- For the reaction, R rarr P, the concentration of a reactant changes fr...
Text Solution
|
- In a reaction 2A to Products, the concentration of A decreases from 0...
Text Solution
|
- For a reaction,A +B to Product : the rate law is given by r =k [A]^(1/...
Text Solution
|
- The convertion of molecules X to Y follows second order kinetics. If c...
Text Solution
|
- A first order reaction has a rate constatn 1.15 xx10^(-3) s ^(-1). How...
Text Solution
|