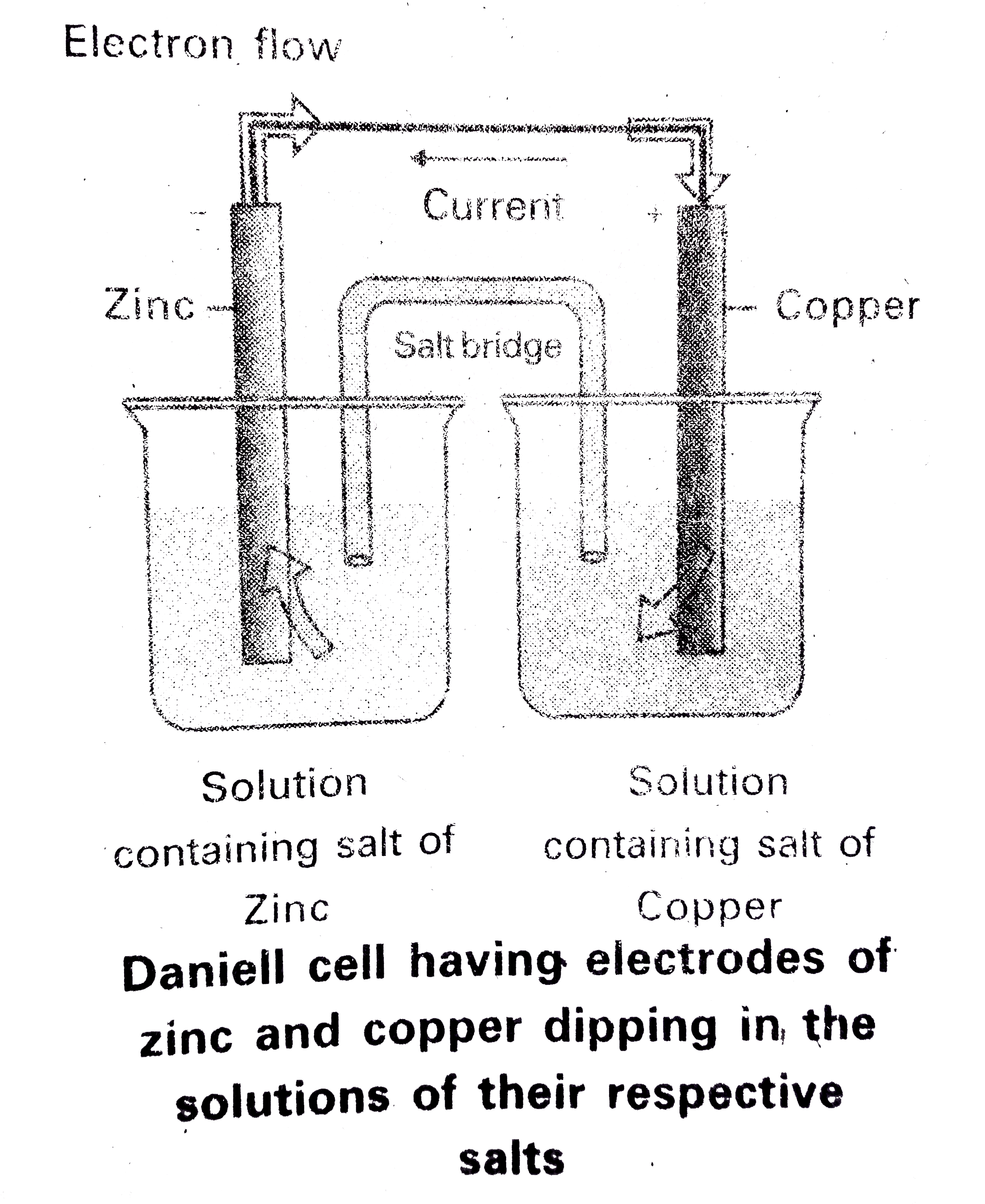Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|24 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|25 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-LONG ANSWER QUESTIONS
- What are electro chemical cells ? How are they constructed ? Explain t...
Text Solution
|
- What is electrical conductance of a solution ? How is it measured expe...
Text Solution
|
- Give the applications of Kohlracsch's law of independent migration of ...
Text Solution
|
- Given the different types of batteries and explain the construction an...
Text Solution
|
- Explain the terms with suitable exapmples. Average rate of a reactio...
Text Solution
|
- Explain the terms with suitable exapmples. Slow and fast reactions
Text Solution
|
- Explain the terms with suitable exapmples. Order of a reaction
Text Solution
|
- Explain the terms with suitable exapmples. Molecularity of a reactio...
Text Solution
|
- Explain the terms with suitable exapmples. Activation energy of reac...
Text Solution
|
- Give two examples for each of zero order and first order reactions. Wr...
Text Solution
|
- Discuss the effect of temperature on the rate of a reaction. Derive ne...
Text Solution
|
- Give a detailed account of the collision theory of reaction rates of b...
Text Solution
|