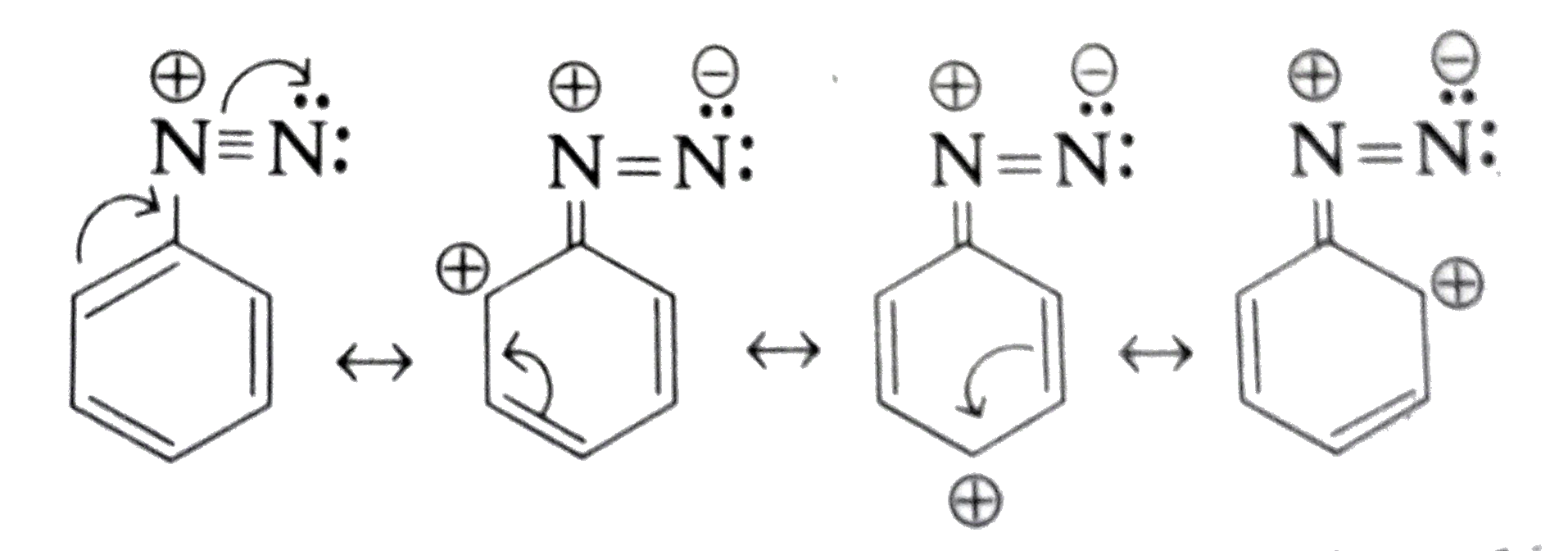
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 4 Marks)|10 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|30 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING NITROGEN-DAM SURE (SAQ - 8 Marks)
- Explain the following name reactions : Sandmeyer reaction
Text Solution
|
- Explain the following name reactions : Gatterman reaction
Text Solution
|
- Complete the following conversions. CH(3)NC+HgO to ?
Text Solution
|
- Complete the following conversions. ? 2H(2)O to CH(3)NH(2)+HCOOH
Text Solution
|
- Complete the following conversions. CH(3)CN+C(2)H(5)MgBrto? overset(...
Text Solution
|
- Complete the following conversions. CH(3)CH(2)NH(2)+CHCl(3)+KOH over...
Text Solution
|
- Complete the following conversions.
Text Solution
|
- Explain why aniline in strong acidic medium gives a mixture of Nitro a...
Text Solution
|
- Complete the following conversions : Aniline to Fluorobenzene
Text Solution
|
- Complete the following conversions : Aniline to Cyanobenzene
Text Solution
|
- Complete the following conversions : Aniline to Benzene
Text Solution
|
- Complete the following conversions : Aniline to Phenol
Text Solution
|
- Account for the stability of aromatic diazonium ions when compared to ...
Text Solution
|
- Write the equations showing the conversion of aniline diazoniumchlorid...
Text Solution
|
- Write the steps involved in the coupling of Benzene diazoniumchloride ...
Text Solution
|