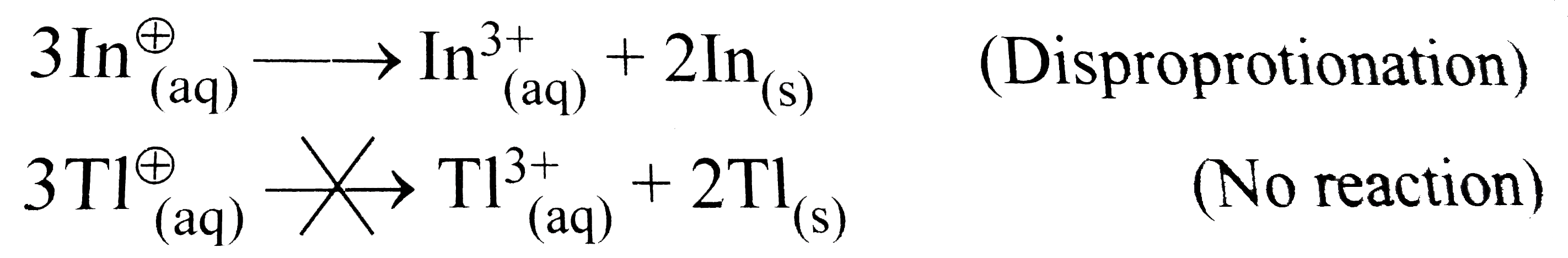Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Ex 6.1 (Objective)|14 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Linked Comprehension)|37 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Solved Examples|16 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|6 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 13 - BORON FAMILY-Ex 6.1 (Subjective)
- Why boron does not exist as B^(2+) ion in solution or in compound ?
Text Solution
|
- The +1 oxidation state is more stable than +3 oxidation state for thal...
Text Solution
|
- Aluminium chloride exists as dimer. Give reason.
Text Solution
|
- Why boron halides do not exists as a dimer. While AlCl(3) exists as Al...
Text Solution
|
- Give reaction for the following : (a) In (III) is more stable than i...
Text Solution
|
- Why boron and aluminium tend to for, covalent compounds ?
Text Solution
|
- Molten aluminium bromide is a poor conductor of electricity. Give reas...
Text Solution
|
- Why B-X bond distance in BX(3) is shorter than theoretically expected ...
Text Solution
|
- Boric acid can be titrated against sodium hydroxide using phenolphthal...
Text Solution
|
- Which one is more soluble in diethyl ether : anhydrous AlCl(3) or hydr...
Text Solution
|
- How is boron obtained from borax ? Give the chemical reactions involve...
Text Solution
|
- Unlike ordinary fire, thermite reaction cannot be stopped by pouring w...
Text Solution
|
- (a) Explain why BF(3) exists whereas BH(3) does not. (b) Compare the...
Text Solution
|
- State with equations what happens when borax is heated on a platinum w...
Text Solution
|
- What is inorganic benzene ? Why is it so called ? How will you get it ...
Text Solution
|
- AlF(3) is insoluble in anhydrous HF but dissolves on addition of NaF. ...
Text Solution
|
- What do you understand by : (a) Ammonal (b) Bentonite ( c) Rubie...
Text Solution
|