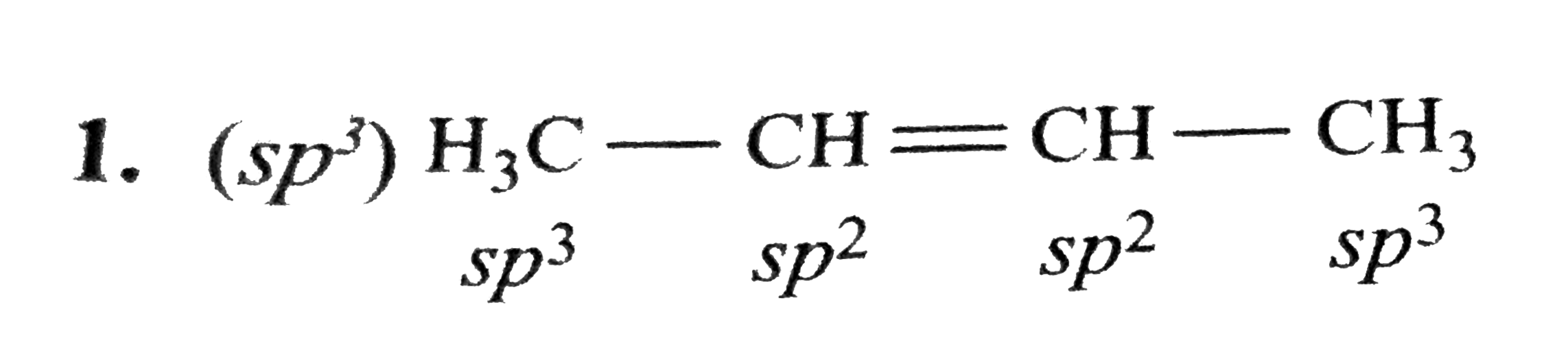
Text Solution
Verified by Experts
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise True/False|1 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|18 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Archives(ASSERTION-REASIOING)|4 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems