Text Solution
Verified by Experts
Topper's Solved these Questions
METALLURGY
TARGET PUBLICATION|Exercise GIVE REASONS|11 VideosMETALLURGY
TARGET PUBLICATION|Exercise GIVE BALANCED CHEMICAL EQUATION|13 VideosMETALLURGY
TARGET PUBLICATION|Exercise MATCH THE FOLLOWING|4 VideosCHEMICAL REACTIONS AND EQUATIONS
TARGET PUBLICATION|Exercise Give balanced chemical equation|26 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Chapter Assessment|9 Videos
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-METALLURGY-ANSWER THE FOLLOWING
- Describe Bayer's process for concentration of bauxite ore.
Text Solution
|
- Explain Hall's process used for concentration of bauxite ore.
Text Solution
|
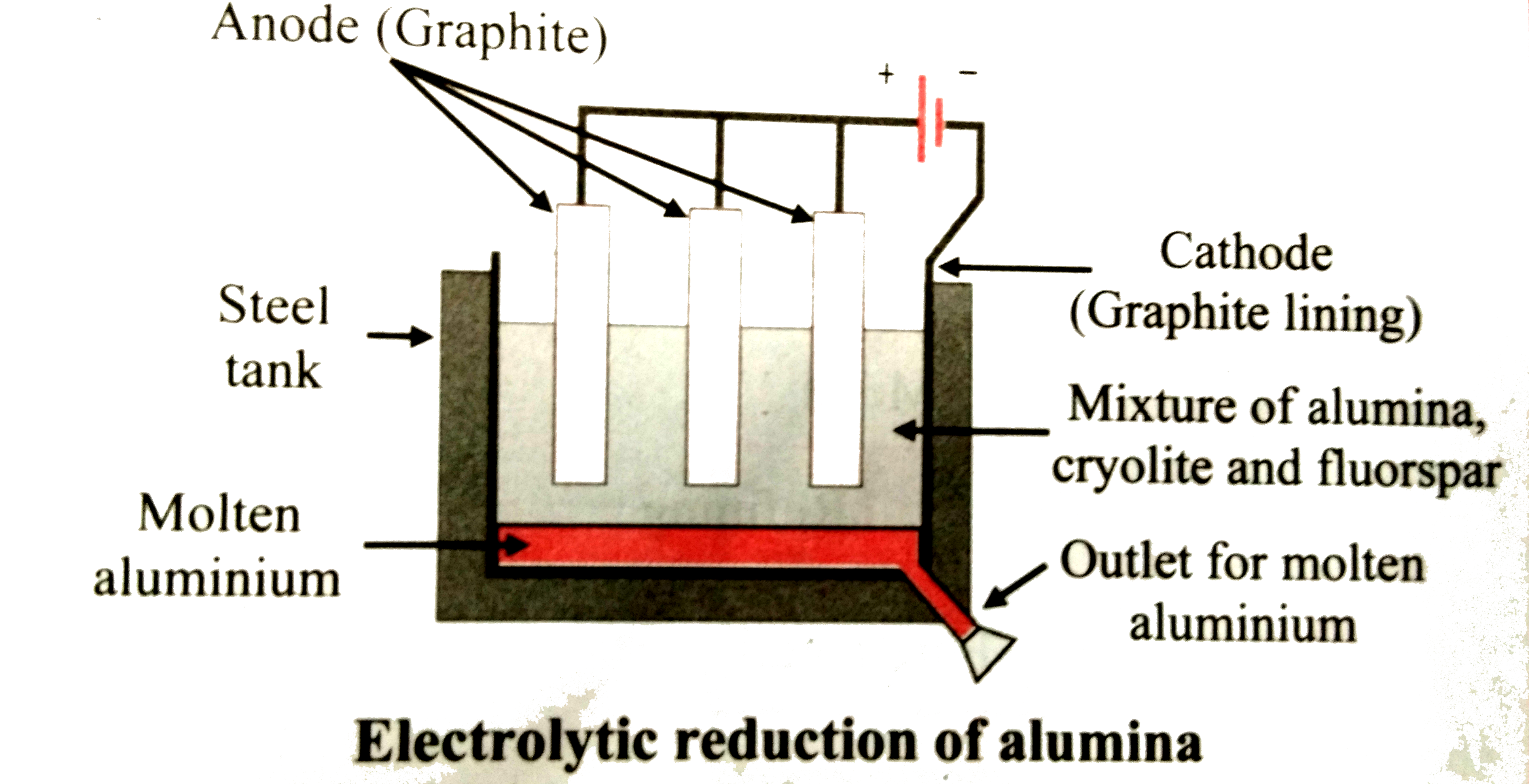
- Describe the electrolytic reduction of alumina with neat labelled diag...
Text Solution
|
- Complete the following statement using every given options. During ...
Text Solution
|
- What are the moderately reactive metals?
Text Solution
|
- In which form do the moderately reactive metals occur in nature?
Text Solution
|
- Why should the metal sulphides and carbonates be converted to metal ox...
Text Solution
|
- Mention the steps carried out for the extraction of zinc from its carb...
Text Solution
|
- Giving examples differentiate between roasting and calcination.
Text Solution
|
- Give any four examples of moderately reactive metals.
Text Solution
|
- 3MnO2 + 4Al to 3Mn + 2Al2O3 + Heat Identify the substances undergo...
Text Solution
|
- What is a thermite reaction? Explain with the help of an equation.stat...
Text Solution
|
- How is copper metal extracted from its sulphide ore ?
Text Solution
|
- Draw a concept map to show the steps to extract metals of medium and l...
Text Solution
|
- State the difference between highly reactive metals and less reactive ...
Text Solution
|
- Give two examples of less reactive metals
Text Solution
|
- REFINING OF METALS
Text Solution
|
- Why do new iron sheets appear shiny?
Text Solution
|
- What is galvanization? What purpose is served by it?
Text Solution
|
- Write a short note on: Tinning
Text Solution
|